Microwave Ablation
If you have kidney, liver or lung cancer, microwave ablation may be part of your treatment. Our multidisciplinary, interventional radiology team* works closely with your referring physician to ensure you receive the best treatment to meet your needs. In addition, our clinical nurse coordinators assist you in planning, scheduling and following up after your treatment.
What is microwave ablation?
Microwave ablation uses microwave energy to generate heat to kill and shrink cancerous tumor cells in your kidney, lungs and liver. Northwestern Medicine interventional oncologists place a small microwave antenna inside a tumor to slow the progression of tumor growth or shrink them to the point where they can be safely removed with surgery.
Your physician will use a CT scan to locate the tumor and insert the antenna through a small incision, guiding the probe to the tumor with continued assistance from the CT. Once the energy source is activated, the heat shrinks and kills cancerous tumor cells, turning them to scar tissue. Because heat also helps close blood vessels nearby, you are typically at a lower risk for blood loss.
Microwave ablation is usually performed as an outpatient procedure.
Benefits and risks
Benefits of microwave ablation include:
- Spares the majority of healthy tissue and can be repeated as often as necessary
- Is well tolerated, and most patients can resume a normal routine within a few days
- Is less painful and easier on you than open surgery or systemic therapy to remove tumors
- Can alleviate pain and other debilitating symptoms caused by tumors
- Uses image guidance, so can provide precise areas for ablation or burning
- Can be combined with other treatment options when needed
- Can be used to reduce the size of your tumor, so that it can be more easily eliminated by chemotherapy or radiation
There are risks following microwave ablation. After the procedure, some patients may experience a small amount of pain or discomfort at or near the area treated. Also, any procedure where the skin is penetrated carries a risk of infection.
Depending on the area being treated, different risks exist, including:
- Liver: Treatment may cause inflammation of the gallbladder, damage to the bile ducts or to the bowel.
- Kidney: Treatment can damage the urine collecting system or cause heavy bleeding.
- Lungs: Treatment may cause a collapsed lung (pneumothorax) or fluid to accumulate around the lungs.
In addition, some patients may experience post-ablation syndrome, which causes flu-like symptoms that appear three to five days after the procedure and usually last about 5 to 10 days.
These risks are rare. Discuss any concerns you have with your physician. Medication can be provided if needed to help prevent or relieve any pain from the procedure.
How Microwave Ablation works
Tissue Heating
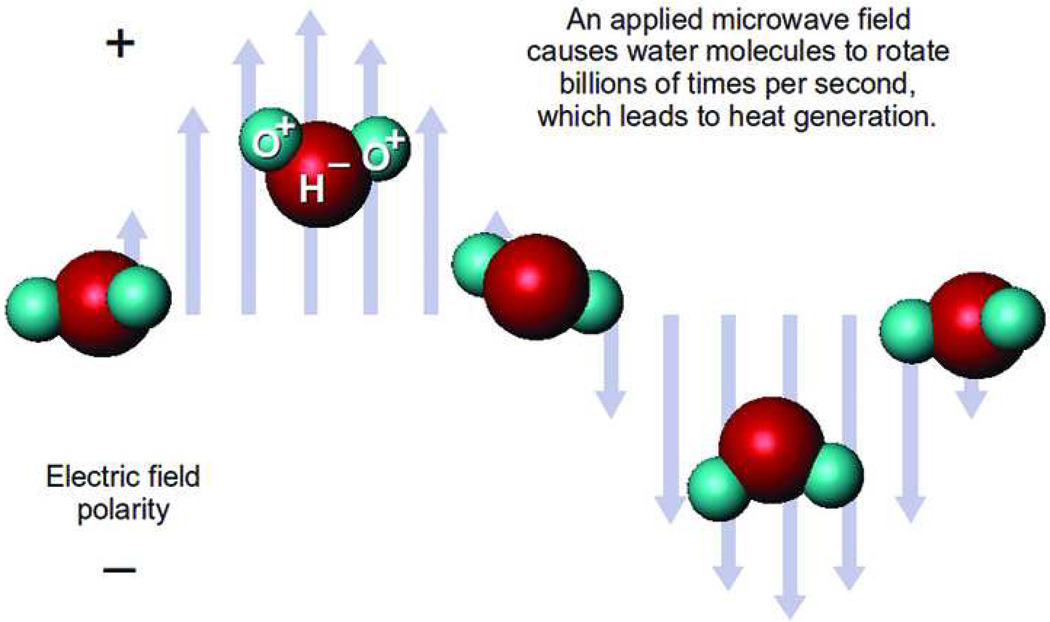
Microwave ablation utilizes dielectric hysteresis to produce heat. Tissue destruction occurs when tissues are heated to lethal temperatures from an applied electromagnetic field, typically at 900–2500 MHz. Polar molecules in tissue (primarily H2O) are forced to continuously realign with the oscillating electric field, increasing their kinetic energy and, hence, the temperature of the tissue (Figure 1). Tissues with a high percentage of water (as in solid organs and tumors) are most conducive to this type of heating.
Microwave heating physics. Schematic demonstrates how an alternating electromagnetic field causes polar molecules to continuous realign, producing kinetic energy and in turn, heat. Reprinted from Curr Probl Diagn Radiol, 38(2), Brace C, Microwave Ablation Technology: what every user should know, 61–67, 2009 with permission from Elsevier.
Microwave energy radiates into the tissue through an interstitial antenna which functions to couple energy from the generator power source to tissue. Due to the radiating nature of the antenna, direct heating occurs in a volume of tissue around the antenna. This mechanism of heating differs substantially from radiofrequency (RF) ablation, which creates heat via resistive heating when electrical current passes through the ionic tissue medium. RF heating requires an electrically conductive path, is limited in areas of low electrical conductivity, and only results in heating of tissues immediately adjacent to the electrode . Microwaves are capable of propagating through and effectively heating many types of tissue, even those with low electrical conductivity, high impedance, or low thermal conductivity. For example, bone and lung are two tissue types that have been associated with suboptimal outcomes or local progression with radiofrequency ablation due to high baseline impedance. Unlike RF and laser, microwaves can readily penetrate through the charred or desiccated tissues which tend to build up around all hyperthermic ablation applicators, resulting in limited power delivery for non-microwave energy systems. Relative permittivity (dielectric constant) is a measure of how well a material or tissue will accept an electric field and is another tissue property that can impact how well energy will propagate through tissue.
Multiple microwave antennas can be powered simultaneously to take advantage of thermal synergy when placed in close proximity, or widely spaced to ablate several tumors simultaneously (Figure 2). Multiapplicator ablation is possible with other power sources, but unlike RF, microwave is able to be powered continuously without switching from one electrode to the others during electrode activation. Also unique to microwave ablation is the ability for antennas to be positioned and phased to exploit overlap of the electromagnetic field . When phased constructively, heating increases proportional to the square of the number of antennas, allowing more efficient heating and generation of higher temperatures when compared to a single antenna. This increase in heating is in addition to the thermal synergy seen with other currently available multiapplicator ablation technologies. In the future, phasing may be able to be exploited in a way that will allow both larger and more customizable ablation zones than is currently possible.
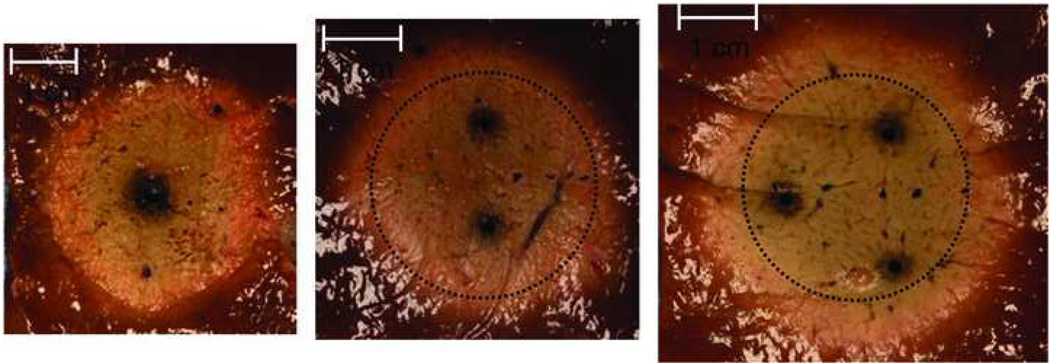
Images obtained during ex vivo ablations with 1, 2 and 3 antennas respectively powered by a 2.45 GHz generator delivering a total of 90 W (1 × 90W, 2 × 45W, or 3 × 30W) for 5 minutes demonstrating the effects of thermal synergy. The dotted circle represents the ablation zone created with a single antenna superimposed on the multi-applicator ablations.
Components of a Microwave System
The basic microwave system consists of three components: a generator, a power distribution system, and antennas. Due to shaft heating caused by reflected power, a cooling system is a crucial component of most microwave antennas and most currently available microwave systems in the US utilize an antenna cooling mechanism.
Power is generated using either magnetron or solid state sources. Microwave generator output can be controlled relatively independent of the tissue type; the impedance spikes or reduced power output characteristic of RF ablation in high impedance tissues are not encountered in microwave ablation. Generator frequencies are generally either 915 MHz or 2.45 GHz, as allowed by the Federal Communications Commission. To date, there is limited data to suggest that any given frequency is more effective for microwave ablation procedures; however, a single preclinical study directly comparing 915 MHz and 2.45 GHz systems has suggested that a 915 MHz generator frequency used in combination with a cooled shaft antenna may generate larger ablation zones than a similar 2.45 GHz system. The 915 MHz ablation zones were also very long and therefore, may be limited in the anatomic areas in which they can be used. Additional study is needed to identify whether these results are reproducible and/or tissue dependent .
Distribution of electromagnetic energy from the generator to the antenna is most commonly accomplished through a coaxial transmission line. Coaxial cables have excellent propagation characteristics, but as cable diameter decreases, power loss (and associated cable heating) increases. Thus, there is a limit to how small and flexible the cables can be without resulting in dangerous cable heating .
Microwave antennas are the final and most critical component of the system, functioning to transfer energy into tissue. The active heating zone and power coupling efficiency of an antenna is determined by its geometry. Most microwave ablation antenna designs are straight and needle-like . Common designs include monopole, dipole, triaxial, choked or slotted antennas .
Microwave antenna design is a balance of power efficiency, tissue heating pattern, and antenna diameter with design tradeoffs necessary to produce a specific desired result. Since antennas are generally constructed from coaxial cable, smaller-diameter antennas can have trouble handling higher powers without unwanted thermal damage to tissues around the proximal antenna shaft (Figure 3 ) . Cooling jackets and antenna shaft cooling systems have been shown to reduce this heating, eliminate skin burns and increase power handling of smaller-diameter antennas.
Shaft heating. Because of the significant shaft heating that can occur with microwaves, a robust shaft cooling mechanism is required to minimize thermal damage to the subcutaneous tissues and the skin, especially with the development of higher power systems.
Circulation of chilled saline or water is the most commonly utilized method for cooling the antenna shaft, and the addition of active cooling has enabled delivery of higher powers for longer times, and in turn, production of larger ablation zones. Another strategy for antenna cooling is the use of compressed gas, utilized by one system (Certus 140, NeuWave Medical, Madison, WI). Rapid decompression of carbon dioxide gas causes the Joule-Thompson phenomenon to occur at the probe tip with gas venting up the shaft and along feed lines. The high cooling capacity of this system allows the use of high power generators (140 W) while maintaining small shaft diameters (17-gauge).
Ultimately, the ablation zone size and shape produced by any antenna in live tissue depends on the antenna design, tissue type (taking into account the changes in the tissue properties during the ablation), thermal conduction from the active heating zone, and thermal sinks caused by nearby structures such as blood vessels. The interplay between these various factors is complex and direct comparisons between the various system designs have not yet been accomplished.
Advantages of Microwave Ablation
Global
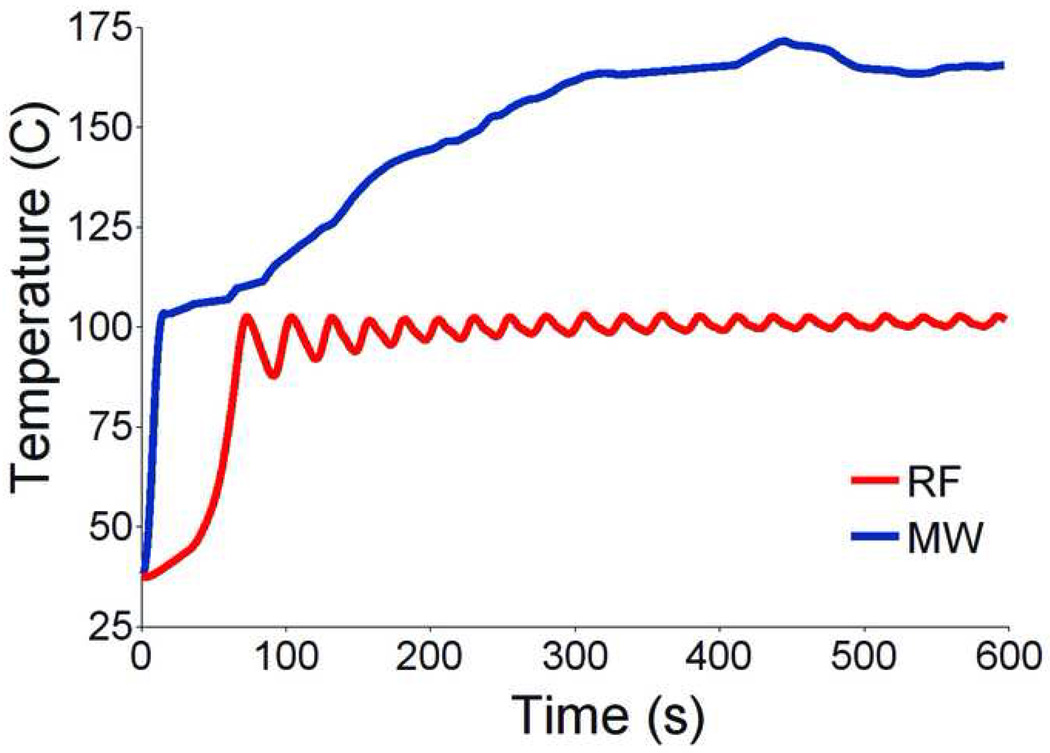
Microwave has many theoretical advantages over current technologies: microwave energy has the potential to produce faster heating over a larger volume of tissue with less susceptibility to heat sink effects; can be effective in tissues with high impedance such as lung or charred, dessicated tissue; is capable of generating very high temperatures, often in excess of 100° C (Figure 4); is highly conducive to the use of multiple applicators; and does not require grounding pads or other ancillary components.
Microwave versus RF temperatures in in vivo porcine kidney. Data collected using a fiberoptic sensor 5 mm away from either an RF electrode (Cool-tip, 200 W, impedance switching) or microwave antenna (triaxial, 50 W, 2.45 GHz) in in vivo normal porcine kidney show higher temperatures (well over 100 degrees C) over time around the microwave antenna .
Microwave also has certain specific organ specific advantages, as detailed below.
Liver
The liver is a vascular solid organ with an abundance of large vessels creating the potential for heat sink effects . Microwaves appear to be more apt to overcome perfusion and large heat sinks than other heat based ablation modalities. Microwave energy has been shown to ablate tissue up to and around large hepatic vessels measuring up to 10 mm and creates larger zones of ablation in high perfusion areas . High perfusion rates in hepatic vessels greater than 3 mm limits the effectiveness of radiofrequency ablation, and has been shown to be an independent predictor of incomplete tumor destruction.
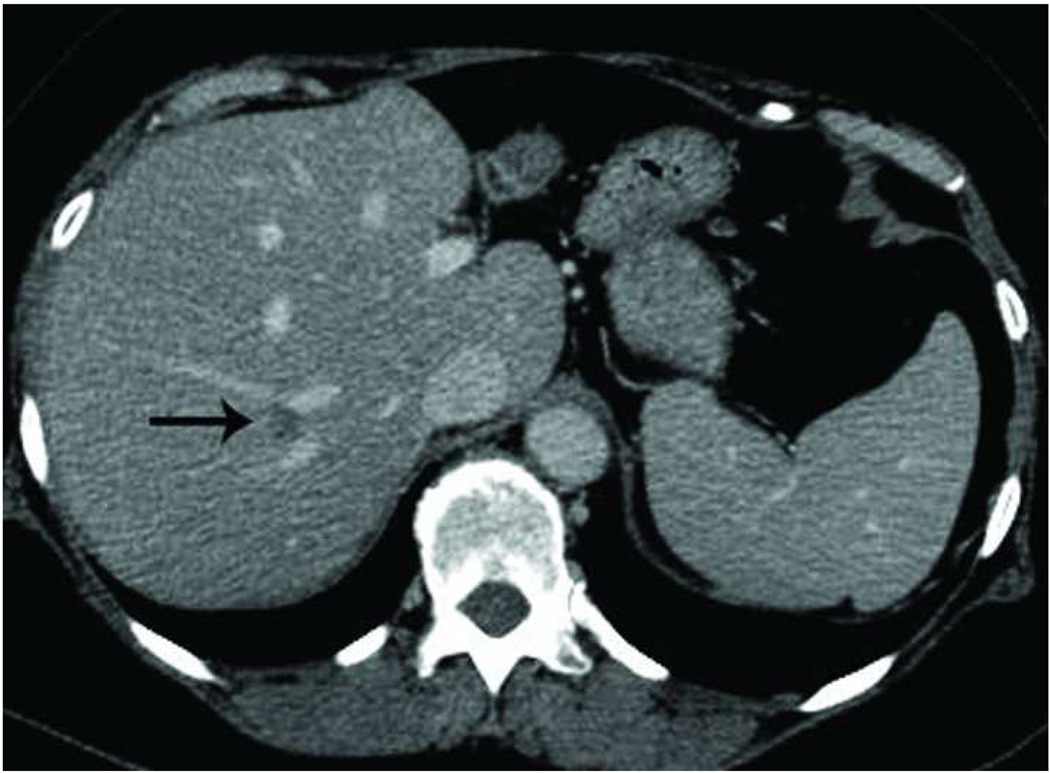
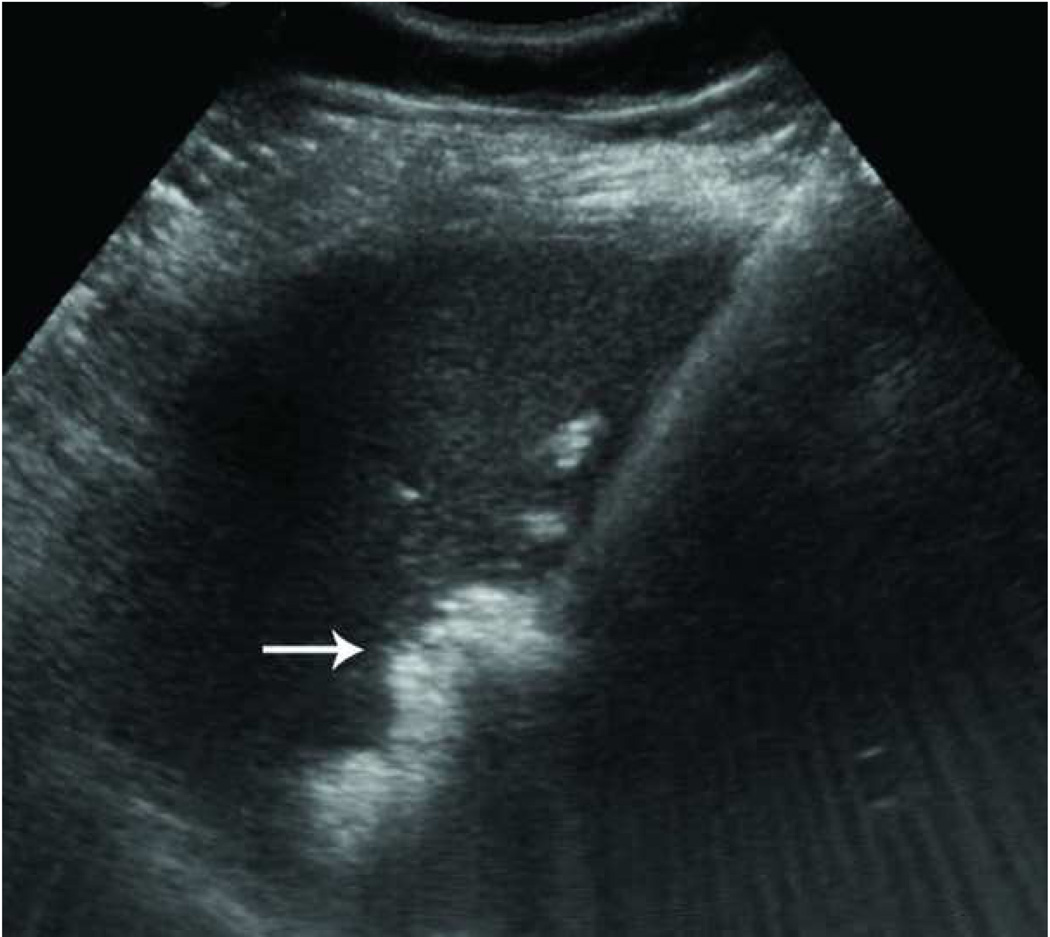
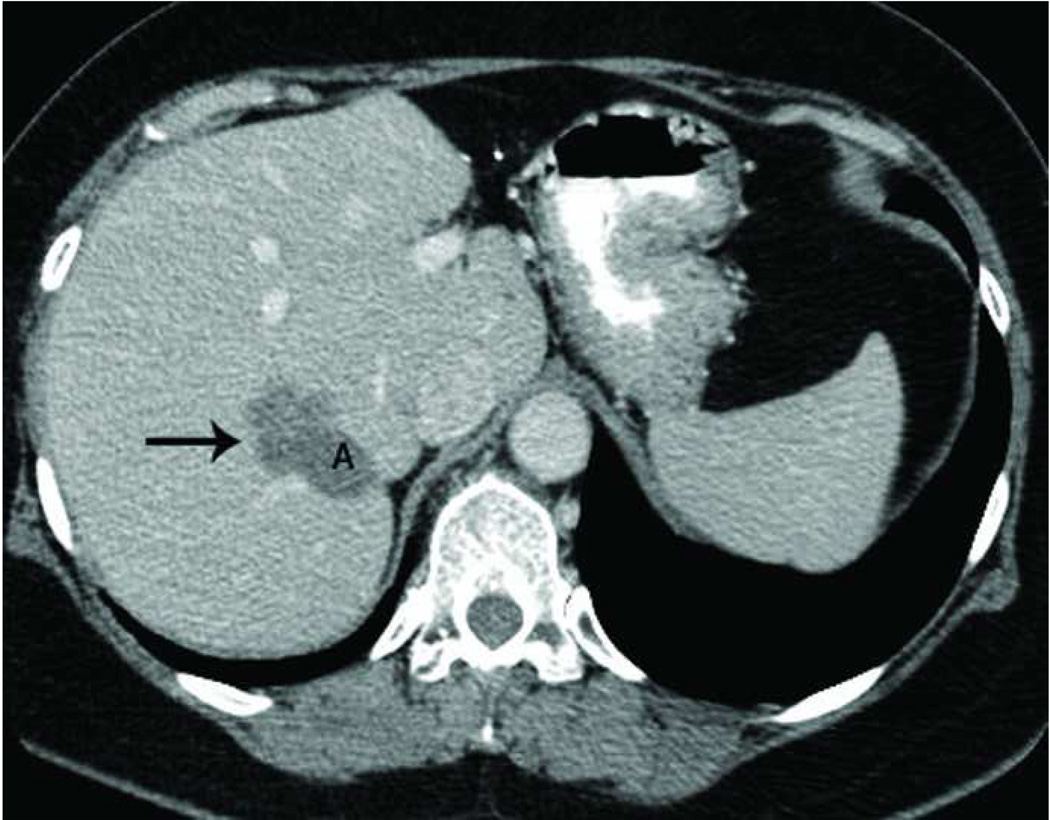
Recurrence along a hepatic vein following RF ablation. Pretreatment CT image (a) demonstrates a small low attenuation lesion located between hepatic venous branches (arrow) concerning for metastatic disease in this patient with colorectal cancer. US image during treatment with RF ablation (b) demonstrates gas encompassing the small lesion. Follow up CT (c) demonstrates a low attenuation ablation zone (A) with local tumor progression along the margin abutting the hepatic vein (arrow).
The decreased susceptibility to vascular cooling has been studied and confirmed in preclinical studies. Awad et al demonstrated large and consistent zones of ablation in shorter times than would normally be seen with radiofrequency ablation and proximity to hepatic vasculature and inflow did not significantly change ablation zone size or shape with microwave ablation . In an in vivo porcine liver model, Brace et al created circular ablation zones with minimal effects related to even large intrahepatic vessels, suggesting that there is minimal heat sink effect near vessels Table 1.
| Study | Year |
# patients |
Treatment |
System parameters |
Tumor size |
Ablation zone size |
Follow up |
LR | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Brace et al | 2009 | 18 | PMWA:9 ablations PRFA: 9 ablations | MWA: 2.45 GHz, 125 W, 17-gauge, 10 min RFA: 200 W, 17-gauge, 10 min | NA, in vivo porcine | MWA: 3.8 +/− 0.3 cm max diameter RFA: 3.3 +/− 0.4 cm | NA | NA | NA |
| Wolf et al | 2008 | 50 | PMWA | 915 MHz, 45–60 W, 14.5-gauge, 5–10 min | Mean diameter 3.5 cm +/− 1.6 | Not reported | Mean=10 mos | 26% residual, 22% recurrence |
1yr-83% 2yr-55% 3yr-45% |
| He et al | 2006 | 12 | PMWA | 2.45 GHz, 30–100 W, 14-gauge, variable time | Range 3–6 cm (lung, mets) | Not reported | Ave=20 mos | Tumors decreased in size | 58% at mean follow up |
| Durick et al | 2005 | 24 | OMWA | 2.45 GHz, 55 W, 17-gauge, 10 min | NA | Mean diameter 3.4 cm (single), 4.8 cm (multiple) | NA | NA | NA |
Abbreviations: LR=local recurrence
PWMA=percutaneous microwave ablation
PRFA=percutaneous radiofrequency ablation
OMWA=open microwave ablation
Clinically, even with early-generation microwave ablation systems, microwave ablation has already been shown in several studies to have equal effectiveness, safety and survival with shorter ablation times when compared to RF ablation for the treatment of small hepatocellular carcinomas . Dong et al looked at 234 patients who underwent percutaneous microwave ablation, and demonstrated favorable survival without severe complications. Lu et al retrospectively compared 102 patients who underwent treatment with either microwave or radiofrequency ablation with no significant difference in survival or complication rates between the two groups .
| Study | Year |
# patients |
Treatment |
System parameters |
Tumor size |
Ablation zone size |
Follow up (mos) |
LR | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Yin et al | 2009 | 109 | PMWA: 50 pts PRFA: 59 pts | MWA: 2.45 GHZ, 60–70 W, 14–16-gauge, 5–20 min RFA: 290 KHz, 200 W, 14–17 gauge, 10–12 minutes | Mean 3.9 cm (20 tumors 5–7 cm) | Mean 22 +/− 18.5 mos |
MWA: 17% RFA: 26.4% |
MWA Median survival: 30.3 mos RFA Median survival: 19 mos |
|
| Shiomi et al | 2008 | 161 | TMWA: 69 PMWA: 73 OMWA: 13 LMWA: 6 | 2.45 GHz, repeated, 14–gauge, 60 W, 60 sec repeats | Mean TMWA= 2.6cm Mean PMWA= 2.4cm | Mean 24.6 +/− 18.5 mos, Median 21 mos, range 1–68 mos | PMWA: 15.1% TMWA: 1.1% | HCC 3 yr PMWA 89.9% TMWA 89.9% CRC mets 3 yr PMWA 5% TMWA 6.6% Other mets 3 yr PMWA 39% TMWA 48% | |
| Ianitti et al | 2007 | 87 | OMWA 42, LMWA 7, PMWA 45 | 915 MHz, 13-gauge, 45 W, 10 min | Mean 3.6 cm (range 0.5–9.0 cm), 22 lesions over 4 cm | Mean single vol 10 ml mean cluster vol 50.5 ml | Mean 19 mos | LR 2.7%, reg rec 43% | Survival 47% at 19 mos (all tumor types) |
| Yu et al | 2006 | 9 | OMWA | 915 MHz, 45–60 W, 13-gauge, 5–10 min | Mean 4.2 cm | Single straight 16.7 cm3; triple straight 51.7 cm3, triple loop 54.3 cm3 | Resect | NA | NA |
| Simon et al | 2006 | 10 | OMWA | 915 MHz, 45–60 W, 13 gauge ×3, 10 min | Mean 4.4 cm; CRC, HCC | Mean diam 5.5 cm, vol 50.8 cm3 | Resect | NA | NA |
| Lu et al | 2005 | 102 | PMWA: 49 pts PRFA: 53 pts | MWA: 2.45 GHz, 10–80 W, 16-gauge, 5 min repeats RFA: 290 KHz, 200 W, 14-gauge multi-tined electrode, 6 min+ |
MWA: 2.5 +/− 1.2 cm RFA: 2.6 +/− 1.2 cm |
MWA: 25.1 +/− 12.7 mos RFA: 24.8 +/− 14.6 mos |
MWA: 11.8% RFA: 20.9% |
MWA: 1yr-81.6% 2yr-61.2% 3yr-50.5% 4yr-36.8% RFA: 1yr-71.7% 2yr-47.2% 3yr-37.6% 4yr-24.2% |
|
| Seki et al | 2005 | 68 | LMWA | 2.45 GHz, 80 W, 45 sec repeats | 1.8 × 2.0 cm | Mean area 42.7 mm2 | Mean 31 mos +/− 16 | 12% | 1yr-97% 3 yr-90% 5yr 48% |
| Liang et al | 2005 | 288 | PMWA | 2.45 GHz, 16-gauge, 60 W for 300 sec, repeats | Mean 3.8 cm +/− 1.6 | Mean 31.4 mos +/− 20.4 | 8% |
Single nodule: 1yr-96% 3yr-78% 5yr-60% Multiple nodule: 1yr-91% 3yr-61% 5yr-28% |
|
| Xu et al | 2005 | 137 | PRFA: 84 PMWA: 53 | RFA: 14-gauge expandable 10 tine, 290 KHz, 200 W, 10 min MWA: 2.45 GHz, 16-gauge, 60W, 5 min | Mean 2.6 cm +/− 1.2 | Mean 24 mos +/− 1.5 |
Overall survival thermal ablation: 1yr 73.9% 3yr 42.8% 5yr 20.1% |
||
| Dong et al | 2003 | 234 | PMWA vs PRFA | 2.45GHz, 10–80 W, 16-gauge, 5–30 min | Mean: 4.1+/− 1.9 cm | 27.9 +/− 18.4 mos | 7.2% |
1yr- 92.7% 2yr-81.6% 3yr-72-8% 4yr-66.4% 5 yr-56/7% |
|
| Shibata et al | 2002 | 72 | PMWA: 36 PRFA: 36 | MWA: 2.45 GHz, 70 W, 16-gauge, 60 sec repeats RFA: 460KHz, 30–60W, 15-gauge expandable, 15 min | MWA: 2.2 cm RFA: 2.3 cm | Range 6–27 months | MWA: 10%, 1 yr; 24% 2 yr RFA: 4%, 1yr; 12%, 2 yr | ||
| Shibata et al | 2000 | 30 | PMWA: 14 Resection: 16 | 2.45 GHz, 60–100W, 16-gauge, 2–20 min | MWA: mean 2.7 +/−1.1 cm Resection: mean 3.4 +/− 1.7 cm (CRC) |
MWA: 1yr-71% 2yr-57% 3yr-14% Resection: 1yr-69% 2yr-56% 3yr-23% |
|||
| Seki et al | 1999 | 15 | PMWA | 80 W, 14–15-gauge, variable time, repeats | Solitary lesions ≤3 cm (CRC) | 9–37 mos | NA | 67% |
Abbreviations: LR=Local recurrence
PMWA=percutaneous microwave ablation
PRFA=percutaneous radiofrequency ablation
TMWA=thorascopic assisted microwave ablation
OMWA=open microwave ablation
LMWA=laparoscopic microwave ablation
CRC=colorectal metastatic disease
HCC=hepatocellular carcinoma
SAD=short axis diameter
More recent studies with newer microwave systems have re-demonstrated the efficacy of microwave ablation in the liver . Shiomi et al compared percutaneous and thoracoscopy-assisted MR-guided microwave ablation in patients with hepatocellular carcinoma and metastatic disease with 3 year survival rates of almost 90% in both groups for patients with hepatocellular carcinoma (median follow up 21 months) . Ianitti et al treated 87 patients with both hepatocellular carcinoma and metastatic disease and found overall survival of 47% (all tumor types) at 19 months .
In addition, preclinical data has suggested that microwaves, particularly with the use of multiple applicators, may be effective in the treatment of larger tumors (> 3cm) . Tumors over 3 cm have historically been problematic for radiofrequency ablation, with a significantly increased risk of local tumor progression . However, the larger ablation zones possible with microwave ablation could potentially make these tumors more consistently treatable. For example, Brace et al demonstrated ablation zones with mean diameters up to 6.5 cm using three 17-gauge microwave antennas spaced 3 cm apart in an in vivo porcine model . Strickland et al used variable times and power outputs ranging from 36 to 200 W in an in vivo porcine liver model and demonstrated ablation zones ranging from 3 to 6 cm in diameter produced very rapidly, i.e. within three minutes .
Early clinical data has supported the hypothesis that microwaves may be more effective against larger tumors . For example, Yu et al later treated four patients with hepatocellular carcinomas greater than 6 cm in diameter, and achieved complete ablation of three of the four lesions in 2–3 sessions . Yin et al treated patients with medium and large hepatic tumors. Although microwave showed a trend toward less local recurrence and larger ablation than for a similarly sized HCC (96% MW, 90% RF, p=0.288), the differences were not statistically significant .
Similarly, early clinical studies have suggested microwaves are effective in the treatment of colorectal hepatic metastatic disease, which requires a larger ablation margin and, hence, a larger ablation zone than for HCC . Shibata et al prospectively randomized 30 patients with multiple metastatic colorectal tumors to microwave ablation or surgical resection and identified no significant difference between the 1, 2 and 3 year survival rates, with less blood loss in the microwave group . Ogata et al treated 102 unresectable colorectal metastatic lesions, with a high local control rate of 95% over median follow up of 33 months. However, new hepatic lesions or extrahepatic recurrence occurred in 78% of patients, and median survival time was 43 months.
Most authors report shorter ablation times in the liver with microwaves than with radiofrequency ablation, frequently less than 10 minutes, and many ranging from 2 to 5 minutes depending on number of applicators, lesion size and power output. From a practical standpoint, decreased time needed for microwave ablation translates to more efficient use of equipment and personnel and decreased time for patients under general anesthesia, which is routinely used for ablation at our institution. However, just as with radiofrequency and cryoablation, use of general anesthesia may vary from site to site depending on physician preference. In addition, the speed of treatment gives microwaves an advantage for treating multiple lesions during one ablation session.
Kidney
The kidney is a highly vascular solid organ with high central perfusion rates creating significant heat sink effects . Combined with concern for damaging the renal pelvis and ureter, large or centrally located tumors can be difficult to treat with RF ablation . The improved ability of microwaves to overcome the cooling effects of blood flow enables production of larger ablation zones in the kidney, which has already been demonstrated in animal studies . Laeseke et al compared microwave ablation with high power triaxial antennas to RF ablation with similarly sized internally cooled electrodes in an in vivo porcine kidney model. Microwave ablation created significantly larger ablation zones with higher tissue temperatures (123 degrees C vs 100 degrees C) .
Early clinical studies have shown that microwave may be effective for the treatment of renal cell carcinoma (RCC). Clark et al operatively treated 10 patients with large renal tumors (3.9–13 cm in diameter) with up to three 13-gauge microwave antennas powered at 60 W for 10 minutes prior to radical nephrectomy. Pathology with viability stains demonstrated an average ablation zone volume of 105 cm3 (5.7 × 4.7 × 3.8 cm) when using a three probe array. An important additional finding was that there was uniform cell death in the ablation zone . Liang et al treated 12 patients with renal cell carcinomas (1.3–3.8 cm in diameter) with microwave and found complete ablation in a single session with no residual or recurrent tumor at a median follow up of 11 months . Although these preliminary results are promising, further clinical experience with the kidney is needed.
Lung
In the lung, less invasive treatment modalities, particularly thermal ablation, have been increasing in popularity for medically unresectable lung tumors, both as a primary treatment and as an adjuvant to external radiation. However, aerated lung has low electrical conductivity and poor thermal conduction, limiting the effectiveness of RF energy . Microwaves, on the other hand, may offer significant advantages in the lung. The low conductivity and high impedance of aerated lung does not degrade the volume heating of microwaves. In fact, the lower permittivity and conductivity inherent in lung may allow deeper microwave penetration than is seen in the liver.
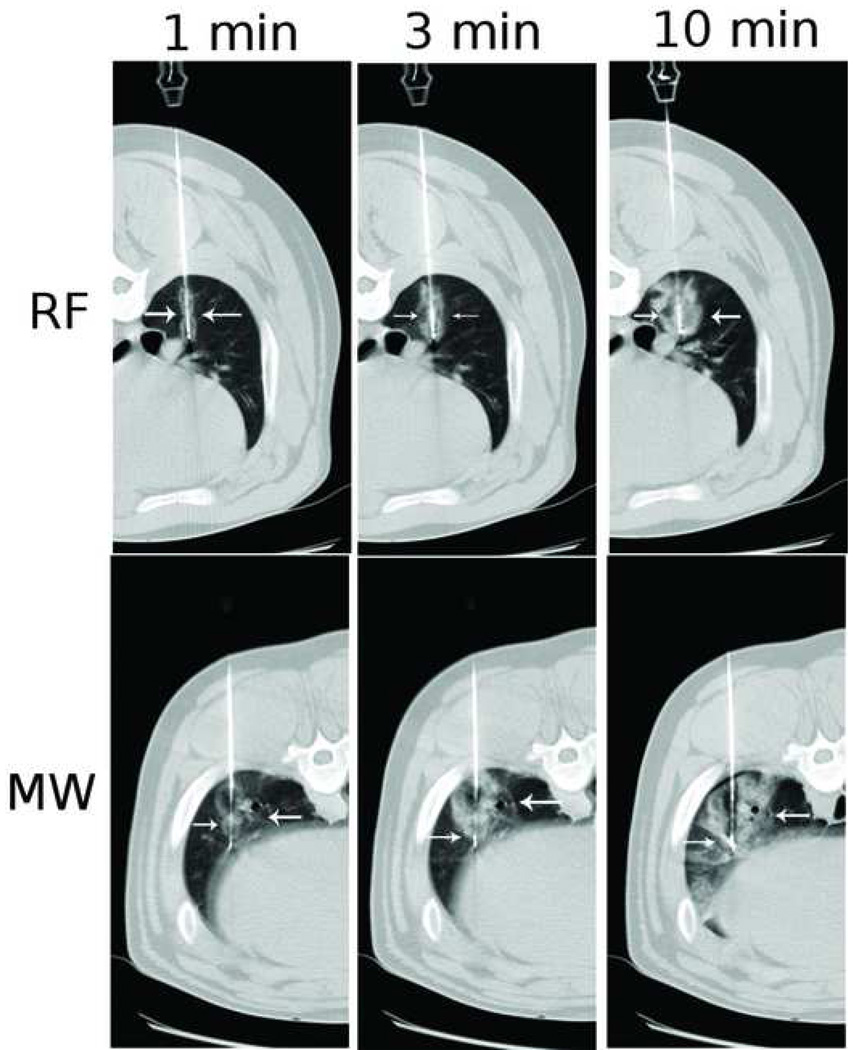
Microwaves have been shown to actively heat larger volumes of normal lung than comparable RF devices . Durick et al showed that with a microwave ablation system tuned for lung, large circular ablation zones in in vivo porcine lungs could be obtained with a single antenna. Coagulation zones created with bronchial occlusion and multiple antennas were significantly larger than those seen without bronchial occlusion and with a single antenna respectively. In a subsequent comparative trial, Brace et al compared ablation zones obtained with a 17-gauge microwave antenna with those obtained with a 17-gauge RF electrode in an in vivo normal porcine lung model. Ablation zones generated using microwaves were 25% larger in mean diameter, significantly more circular and developed faster than those achieved with RF energy .
Sequential CT images obtained during RF vs MW ablation in in vivo porcine lungs. Note the larger ablation zones (arrows) at each time point in the microwave panel. Reprinted from Radiology, 251(3), Brace C, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT Jr, Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model, 705-11, 2009 with permission .
| Study | Year | Model |
# treatments |
Treatment |
System parameters |
Ablation zone size |
|---|---|---|---|---|---|---|
| Brace et al | 2007 | in vivo porcine | 58 | OMWA | 2.45 GHz, 68 W, 17-gauge, 2–12 min | Mean diam: 2 min-2.3 cm 6 min-2.6 cm 12 min-2.9 cm |
| Awad et al | 2007 | in vivo hepatic porcine | 6 | OMWA | 2.45 GHz, 100 W, 5.7 mm antenna, 2–8 min | Mean vol: 2 min-33.5 cm3 4 min-37.5mm3 8 min-92 cm3 |
| Hines-Peralta et al | 2006 | ex vivo bovine, in vivo porcine | 165 (120 ex vivo bovine, 45 in vivo porcine) | OMWA | 2.45 GHz, 50–150 W, 5.7 mm antenna, 2–20 min | Ex vivo 4.9 cm SAD In vivo 5.7 cm SAD |
Note that none of these experimental protocols utilize a lung tumor model and the properties of lung tumors may be more similar to those of solid organs. However, early clinical studies suggest that tumor heating with microwave energy is also effective . In the largest study to date, Wolf et al percutaneously treated 82 lung masses (primary lung tumors and metastatic disease) in 50 patients. Local control rate (based on serial follow up imaging with CT and PET) at one year was 67% with a mean time to first recurrence at 16.2 months . Although preliminary data suggests that microwave is safe and effective in the lung, further study is needed.
Bone
Bone has low conductivity (high impedance) and thermal conduction which serve to limit the effectiveness of RF ablation. Microwave is relatively insensitive to high impedance and has a deeper penetration profile. Both of these characteristics may represent a relative advantage for microwave ablation in this setting. Due to the relative permittivity of bone, microwave may be less affected by tissue heating and desiccation, providing deeper penetration and more effective heating. Two different applications for ablation in bone currently exist: treatment of painful osteoid osteomas, and treatment of other bone tumors, particularly painful metastases. There is very little experience with treatment of osteoid osteomas with microwaves, perhaps due to the fact that a microwave system capable of producing short ablation zones (~1 cm) is not yet available. Currently available antennas produce zones of ablation as long as 5–7 cm. Thus, microwaves may not currently have clinical application in the treatment of osteoid osteomas given the risk of non-target thermal damage. Early clinical use of microwave in other bone tumors/metastatic disease has been promising. For example, Fan et al treated 213 patients with malignant bone tumors of the extremities and pelvis with combined operative intervention and microwave ablation therapy in a limb sparing procedure, with a survival rate of 74% at an average follow up of 49 months. Further study is needed in both areas to confirm the theoretical advantages in bone as only a few cases have been published in the literature to date .
Disadvantages of Microwave
Global
Microwave power is inherently more difficult to generate and deliver safely and efficiently to the tissue when compared to RF. This is primarily due to the fact that microwave energy must be carried in coaxial cables which are larger in diameter, more cumbersome, and more prone to heating than the simple wires used in RF ablation. Decreased cable surface area leads to more power loss and increased cable heating. Since one of the primary advantages of microwave is the ability to deliver large amounts of power, the technical hurdles to distribute this power to tissues without significant cable and shaft heating must be overcome before this advantage can be fully realized. A robust active shaft cooling mechanism can mitigate many of these risks and is imperative to high power delivery . A clinical study comparing cooled with non-cooled antennas in a cohort of 1136 patients showed that use of the cooled-shaft antenna led to fewer treatment sessions and fewer major procedural complications .
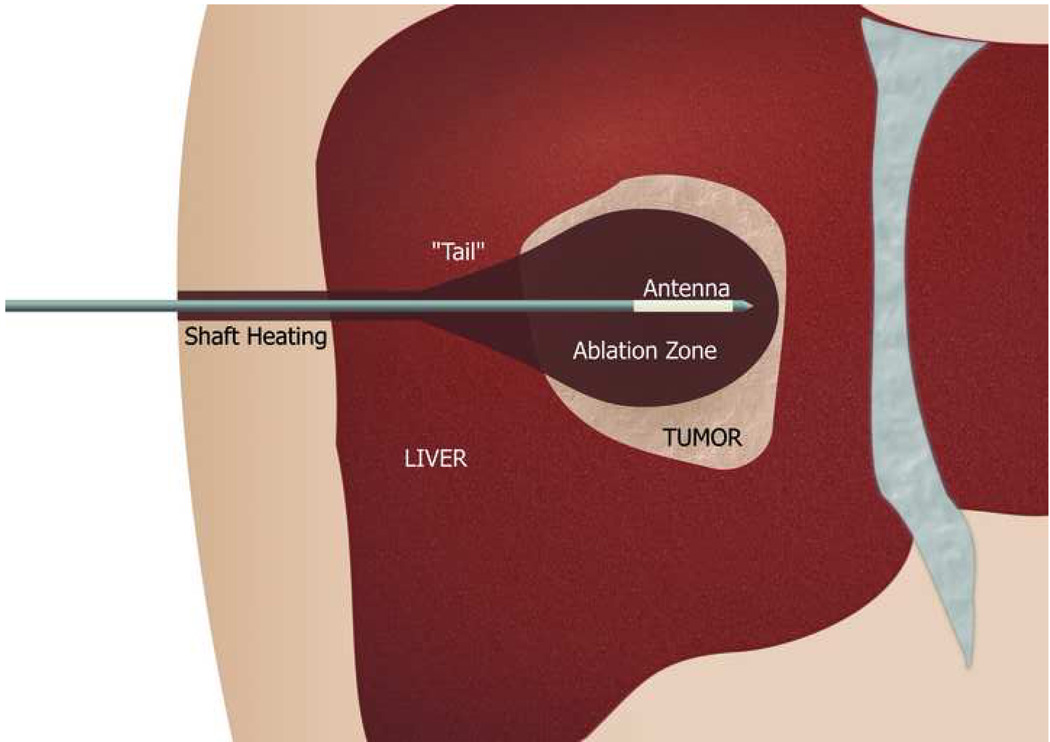
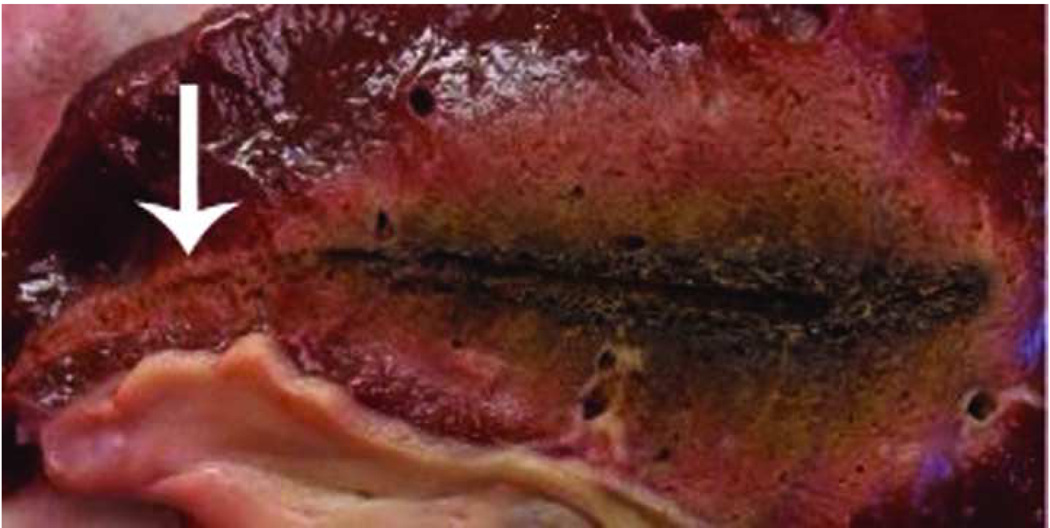
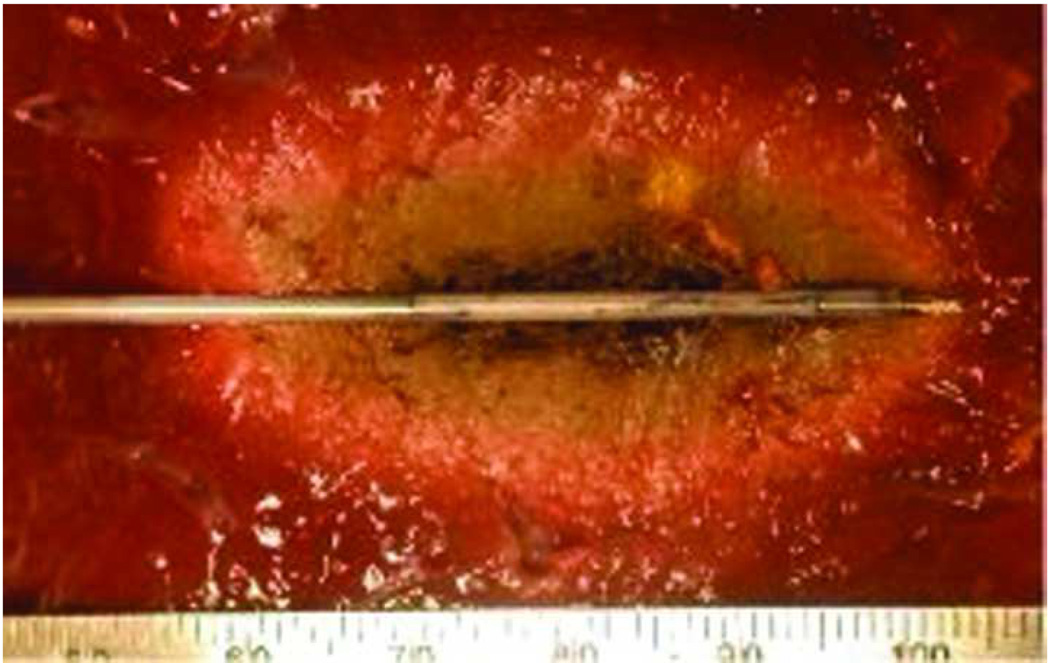
Non-cooled antennas can cause proximal tissue heating, creating an unwanted tail of ablation in the more superficial tissues, as seen in this photograph of a hepatic microwave ablation zone (arrow, a). However, cooling eliminates this unwanted shaft heating and ablation tail, as seen in (b), a hepatic ablation zone generated with an identical power and time but with the addition of water cooling of the antenna shaft.
Currently available microwave systems continue to face technical limitations, and this has prevented the potential of microwave from being realized in the clinic to date. Major limitations include underpowered systems, shaft heating, large diameter probes, long and relatively thin ablation zones which have limited clinical application (especially in small bone lesions such as osteoid osteomas and solid organ surface lesions). Similarly, there is still some unpredictability to the size and shape of the zone of ablation which may be related to technical factors.,9
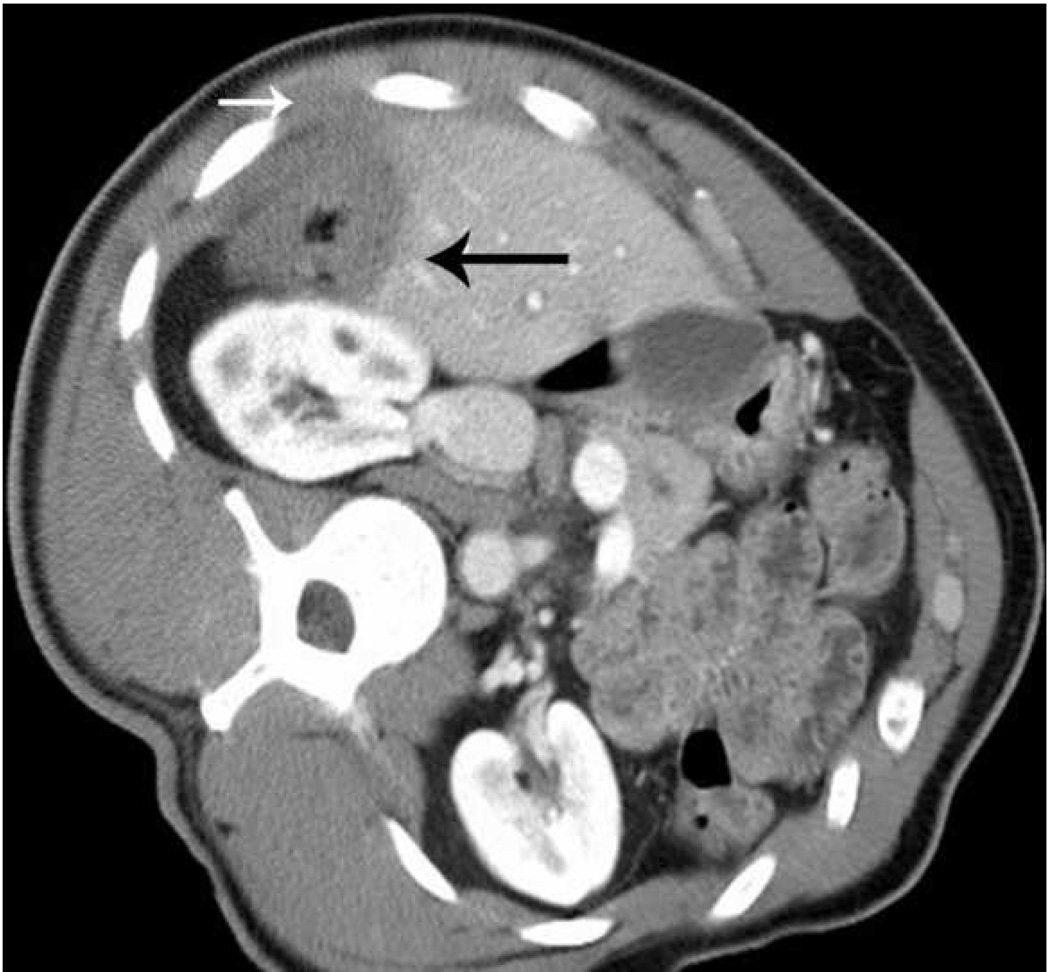
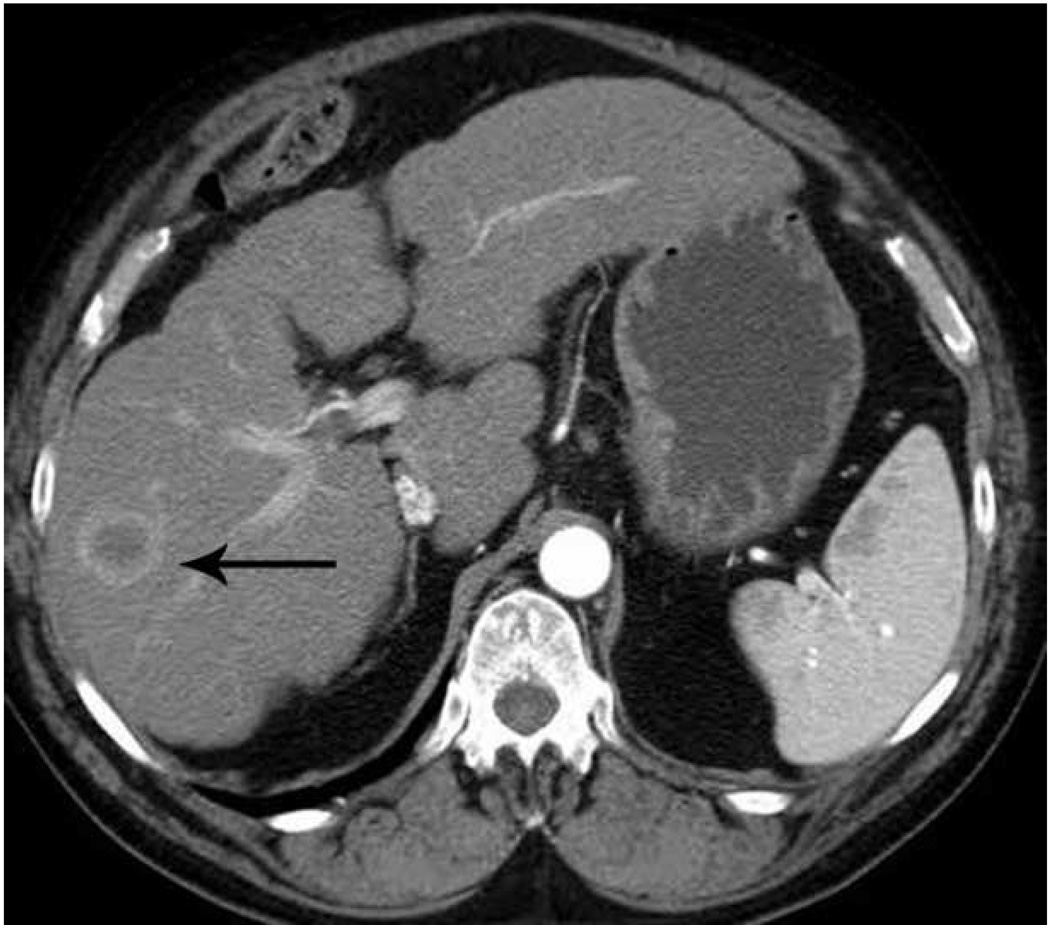
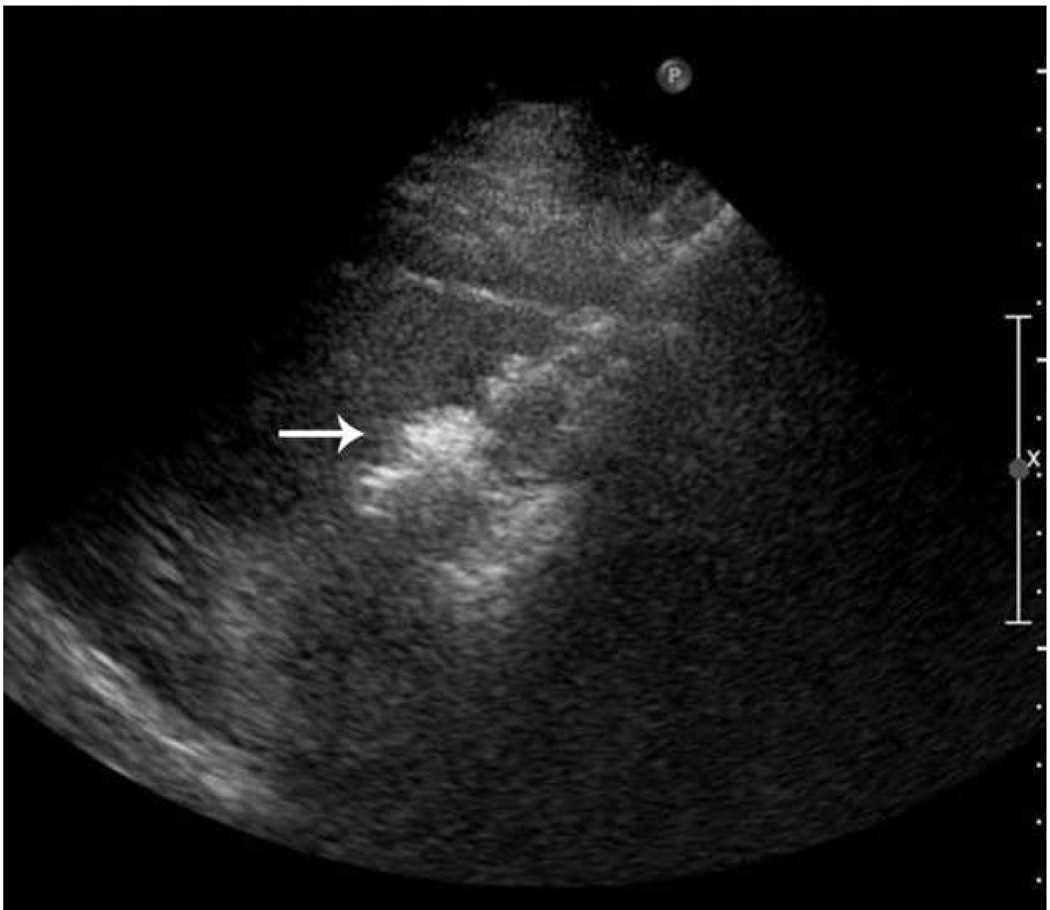
Microwave ablation of small hepatocellular carcinoma using the Covidien Evident™ system. Pre ablation CT (a) demonstrates a small hepatocellular carcinoma (arrow) in a patient with hepatitis C and relatively mild cirrhosis. Two probes were placed, and ablation was performed with a large area of gas bubbles forming on the periprocedural US (b). These probes, run at 45 W for 10 minutes, generated a larger than expected ablation zone (large arrow) that extended into the body wall (small white arrow), seen on post procedure CT (c).
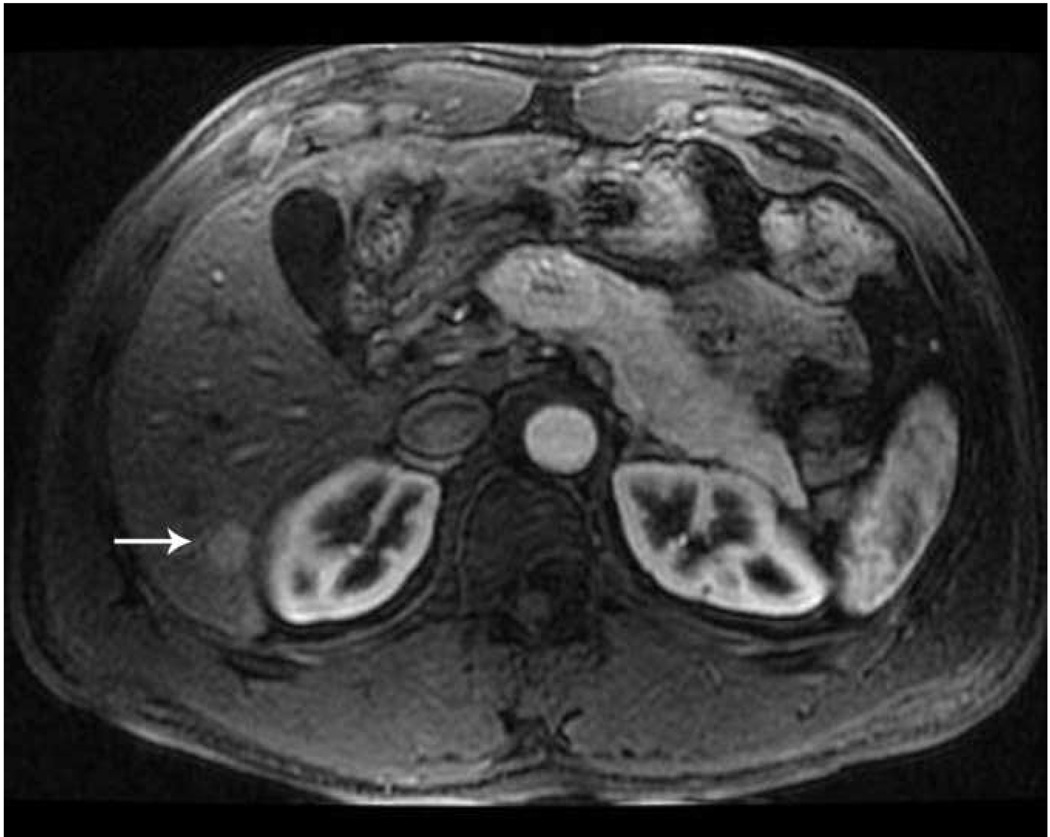
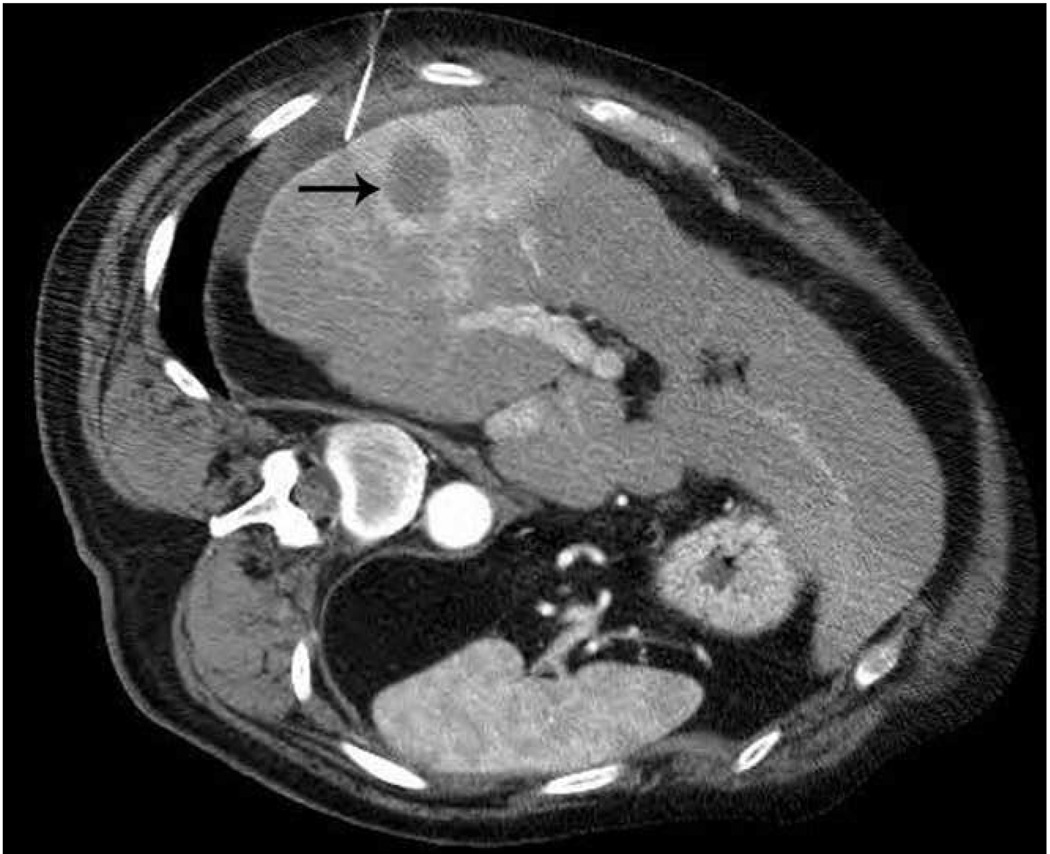
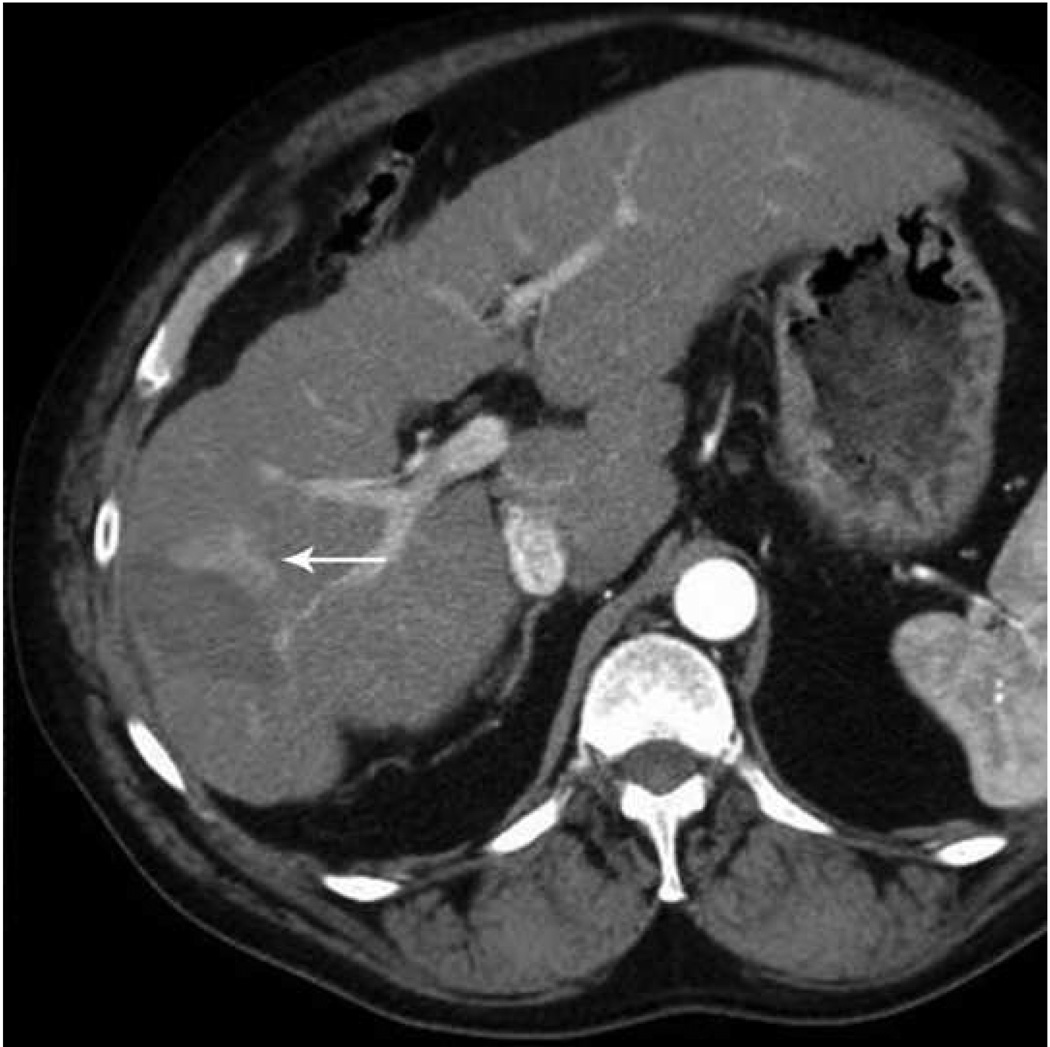
Microwave ablation of liver lesion using Covidien Evident™ system. Pre procedure CT (a) demonstrates a small hepatocellular carcinoma (arrow) in a mildly cirrhotic liver. Two probes (b) were placed into the lesion and run at 45 W for 10 minutes, similar to the case in figure 5. However, a relatively small area of hyperechoic change was identified on the periprocedural US (arrow, b) and the immediate post procedure CT image (arrow, c) demonstrates a much smaller ablation zone with residual tumor seen along the anterior and medial margins at one month follow up (arrow, d). The variation in result between these two cases may be related to increased sensitivity of microwaves to probe placement and phasing.
Note that as detailed above, microwave has theoretical advantages in all tissue types. One tissue specific disadvantage may be seen in bone with osteoid osteomas, as these lesions require a short ablation zone which most microwave systems are not currently capable of generating.
Currently Available Microwave Systems
In the United States, only one FDA-cleared microwave system is widely available for commercial use (ValleyLab/Covidien). The Evident™ system is a 13-gauge water cooled dipole antenna with a 915 MHz generator and a maximum recommended output of 45 W. Preclinical testing was first performed by Wright et al, who demonstrated that simultaneous multi-probe ablations were nearly 6 times larger in volume than single probe ablations . Oshima et al also explored the use of multiple applicators with this system and found that when spaced 2 cm or less, spherical ablation zones were maintained, with significant increase in size of the zone compared to those created with a single antenna. Iannitti et al used this system in a phase II clinical trial to treat 87 patients with hepatic tumors. Mean follow up to 19 months demonstrated local tumor progression seen in 2.7% and regional recurrence/new foci of disease in the target organ in 43%. Martin et al also used this system for a prospective phase II study of microwave ablation of hepatic malignancies (hepatocellular carcinoma and metastatic disease-most treated operatively) in 100 patients. At median follow up of 36 months, 5% of patients had incomplete ablation (seen at initial 2 week follow up imaging), 2% had local tumor progression, and 37% demonstrated intrahepatic recurrence at non ablated sites. These early results are promising, but the system needs more extensive in vivo characterization and validation in larger clinical trials before its full potential will be known ,9.
Other systems that have recently received FDA 510(k) clearance or are currently in development include: the MicrothermX-100™ from BSD Medical (Salt Lake City, UT), which uses a 915 MHz generator and multiple 14-gauge applicators with individual antenna control; MedWaves’ AveCure™ system (San Diego, CA) which uses a 915 MHZ generator and 12–16-gauge antennas with temperature feedback rather than cooling; and the Certus 140™ from Neuwave Medical, a 2.45 GHz system under development which supports up to three actively gas-cooled 17-gauge triaxial antennas (Madison WI).
In Europe, the Acculis Microwave Tissue Ablation system consists of a 2.45 GHz generator, with power output settings ranging from 30–100 W, and a percutaneous 15-gauge cooled antenna. The HS Amica microwave system uses a 2.45 GHz generator, with power output to 100 W (60 W recommended output) through 14-gauge and 17-gauge water cooled applicators .
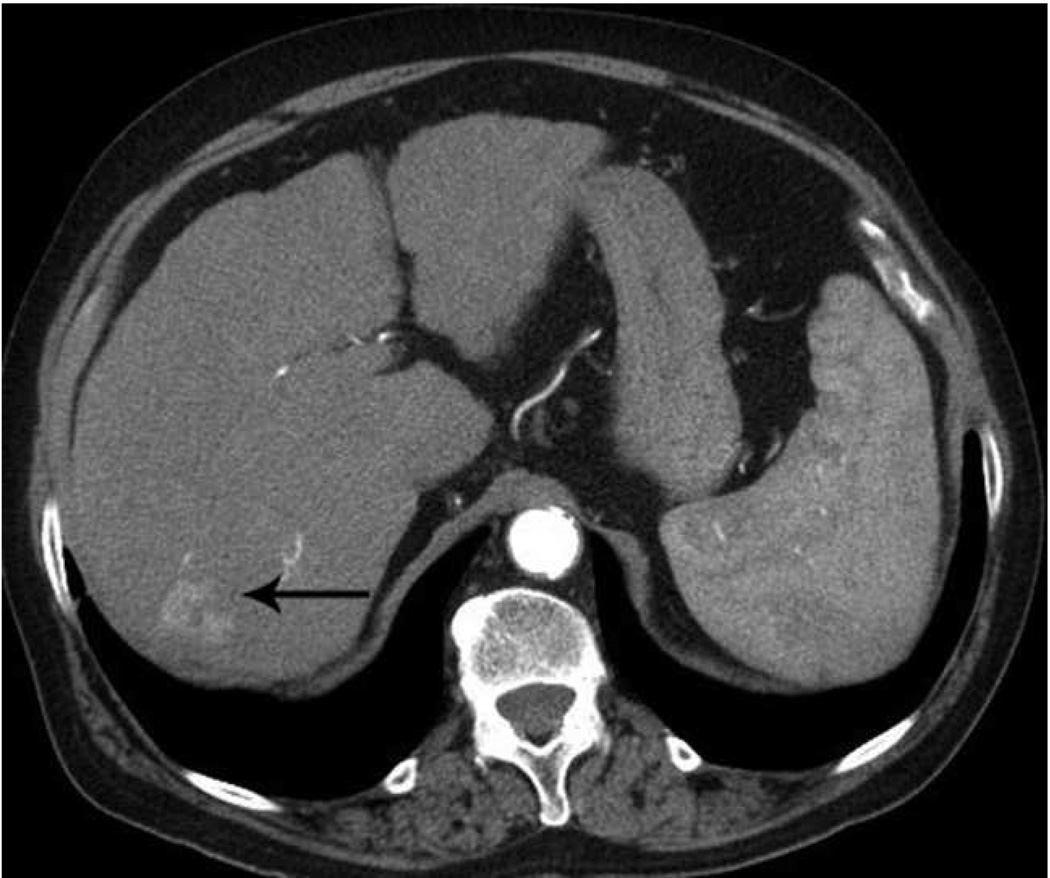
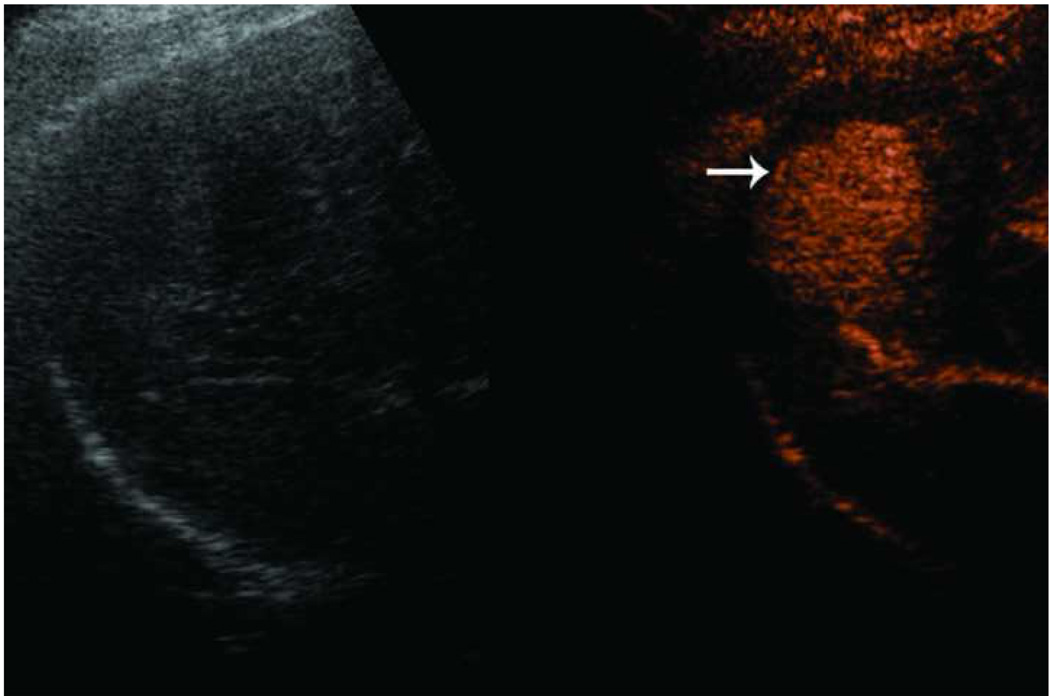
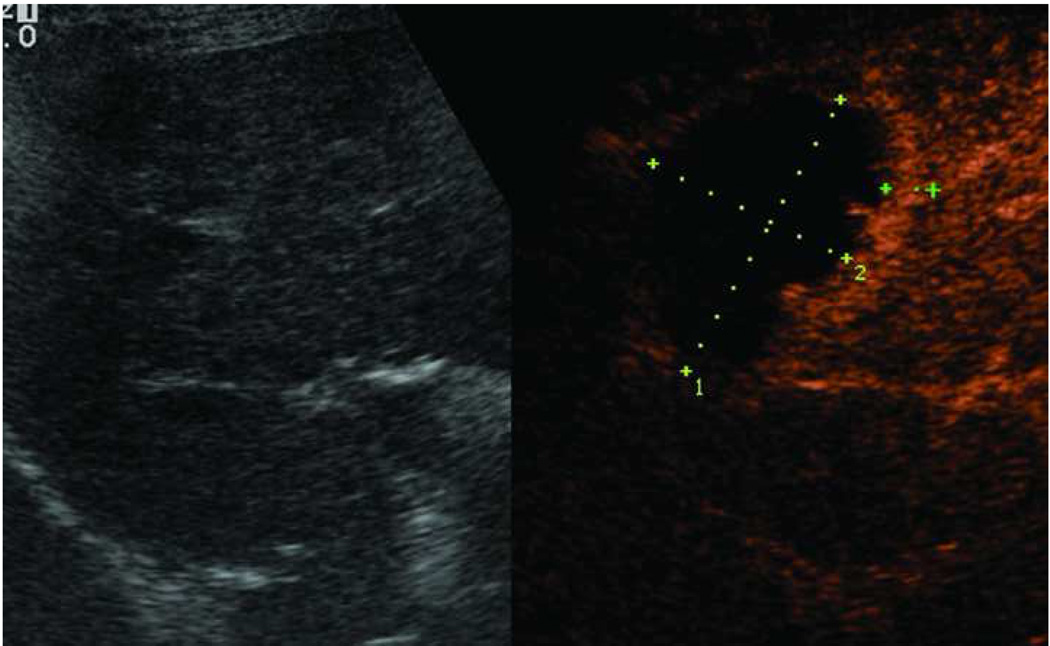
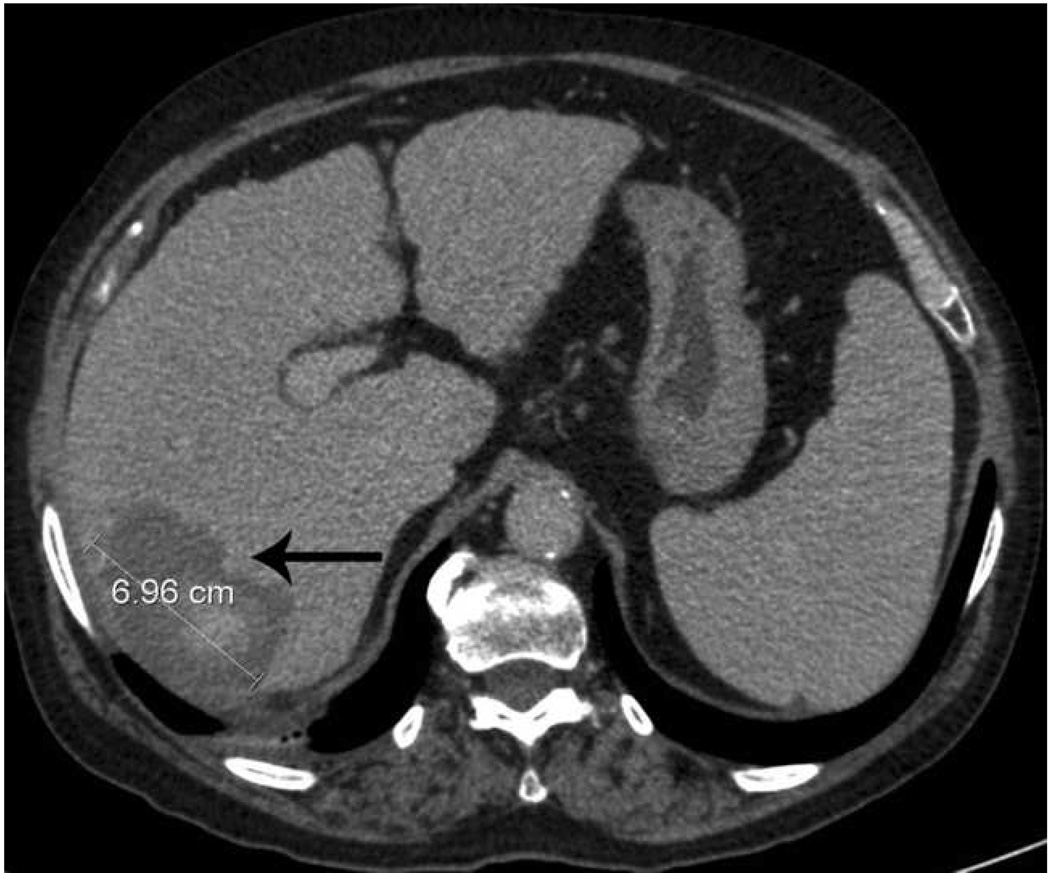
Microwave ablation performed with HS Amica System. Pre-procedure CT demonstrates nodular arterially enhancing lesion in the posterior right hepatic lobe (arrow, a), redemonstrated on contrast enhanced pre-procedure ultrasound (b). Three sequential placements were performed with an antenna powered at 60 W for 15 minutes each. This produced an ablation zone nearly 7 cm in maximal diameter, as seen on post procedure US (c) and CT (arrow, d). Case courtesy of Drs M. Franca Meloni and Anita Andreano, University of Milan-Bicocca.
To date, microwave ablation has been utilized most widely in Japan and China, where multiple systems have been developed. Most of these systems use 2.45 GHz generators with monopole, dipole or slotted coaxial antennas. For example, the FORSEA system (Qinghai Microwave Electronic Institute, Nanjing, China) utilizes a 10–150 W, 2.45 GHz generator and 14-gauge water-cooled antenna. Kang-Yu Medical has both a 915 MHz and a 2.45 GHz generator system (KY2000-915 and KY2000-2,450) with a water cooled 15-gauge antenna. Both generators can produce 1–100 W. The Microtaze (Nippon Shoji, Osaka, Japan) also utilizes a 2.45 GHz generator with a 16-gauge cool shaft antenna with outputs between 60–70 W. For a summary of available systems, see Table 4. Given the substantial limitations in commercially available devices to date, commercial and academic development is ongoing.
| Company |
Antenna design |
Antenna size (gauge) |
Cooling type |
Power (W) |
Frequency |
Multiple applicators |
|---|---|---|---|---|---|---|
| Valley Lab/Covidien | Straight | 13-g | Water | 45 | 915 MHz | N |
| BSD Medical | Straight | 14-g | Water | 60 | 915 MHz | Y |
| MedWaves | Straight | 12 to 16-g | None | 32 (modul ated) | 915 MHz | N |
| Neuwave Medical | Triaxial, straight | 17-g | Gas | 140 | 2.45 GHz | Y (3) |
| Acculis | Straight | 15-g | Water | 100 | 2.45 GHz | N |
| HS amica | Straight | 14-g 17-g | Water | 100 | 2.45 GHz | Y (2) |
| FORSEA | Straight | 14 | Water | 150 | 2.45 GHz | Y (2) |
| Kang-you Medical | Straight | 15 | Water | 100 | 915 MHz, 2.45 GHz | |
| Microtaze | Straight | 16 | Water | 70 | 2.45 GHz |
Conclusion
In summary, microwave tumor ablation is an exciting technique with many theoretical advantages over currently existing ablation systems including generation of larger ablation zones in shorter times with very high temperatures, and less susceptibility to heat sink effects. In addition, microwaves have an advantage in high impedance tissues such as lung and bone that have been problematic for other ablation modalities. Preclinical and early clinical studies have begun to confirm these advantages; but further study and continued development of more robust clinical systems is still needed.