I. Hepatic Arterial Infusion
What is Hepatic Arterial Infusion (HAI)?
HAI is a type of regional chemotherapy to treat advanced colorectal cancer that has spread to the liver. It delivers a high dose of chemo directly into the liver's blood supply using a pump that surgeons implant under your skin.
In most cases, people have already had surgery on their colon or rectum. They've also had systemic chemo through an IV line that travels all through the body.
HAI may:
-
Help some people with liver metastases live longer.
-
Limit negative side effects.
-
Improve quality of life.
Doctors may use other treatments along with HAI therapy, including:
-
Isolated hepatic perfusion (IHP).
-
Yttrium-90 internal radiation.
-
Systemic chemotherapy.
Your doctor will work with you on treatment goals to meet your needs.
Hepatic Arterial Infusion Benefits
Research shows that HAI may help people live longer, especially those with advanced colorectal cancer.
HAI delivers the chemo directly to your cancer cells, limiting how much of the drug reaches healthy parts of your body.
Hepatic Arterial Infusion Risks and Side Effects
Although HAI is less likely to cause side effects like systemic chemo, the possibility of side effects still exists.
These side effects may include:
-
Nausea
-
Vomiting
-
Diarrhea
-
Swelling of the liver
-
Damage to the bone marrow (myelosuppression)
Doctors must perform surgery to install the HAI pump and connect it to your hepatic artery.
Possible risks of any surgery include:
-
Infection
-
Blood loss
-
Blood clots
-
Other problems
Other risks — specific to HAI surgery — are rare but may include:
-
Injury to the artery.
-
Leakage of chemo into nearby organs.
-
Inflammation of the stomach or nearby organs.
-
Pump pocket inflammation or infection.
Cancers We Treat with Hepatic Arterial Infusion
The most common use of HAI is to treat primary liver cancers like cholangiocarcinoma and metastatic colorectal cancer.
How to Prepare for Hepatic Arterial Infusion
Your surgeon will give you specific guidance on how to prepare for your cancer surgery.
These guidelines may include the following:
-
Complete any necessary preoperative testing at least a week before your surgery date. These might include blood and urine tests, a chest x-ray, an EKG, and others as needed.
-
Stop taking aspirin, blood thinners, or anti-inflammatory drugs 10 days before your surgery. Your doctor will let you know if and when you should stop vitamins or other supplements.
-
Stop eating and drinking at least eight hours before your surgery. Your doctor may let you take medicine with a sip of water (no coffee, tea, or juice) the morning of surgery. If you have diabetes, ask whether you should take your diabetes medications on surgery day.
What Can I Expect During and After Hepatic Arterial Infusion?
HAI surgery can be either open or robotic. Your surgeon will discuss these options with you in advance.
Your HAI surgery will start with an arteriogram, an imaging test in which doctors check your liver's arteries to plan the operation.
Next, your surgeon will:
-
Remove your gallbladder, if you still have it.
-
Insert the HAI pump into the artery.
-
Perform a dye injection test to make sure the chemo will go where it's needed.
Your surgery will last between two and a half to three hours.
You can expect a hospital stay of about four days post-op. You may need to stay longer if have any complications after surgery.
Once your surgical wound starts to heal, your doctor may send you home with your first dose of HAI chemo. You will receive more doses as needed during the coming weeks and months, based on the chemo drug you get.
Because HAI is complex, it's best to choose an experienced cancer center for your treatment.
UPMC Hillman Cancer Center's surgeons have more than 20 years of experience with HAI and minimally invasive surgery.
II. Pancreatic intra-arterial infusion chemotherapy
A Patient With Stage III Locally Advanced Pancreatic Adenocarcinoma Treated With Intra-Arterial Infusion FOLFIRINOX
Patients affected by pancreatic ductal adenocarcinoma (PDAC) have very poor prognosis, whereby at a follow-up of 5 years, the mortality rate is very similar to the incidence rate. Globally, around 10% of patients are amenable to radical surgery at the time of diagnosis, which represents the only chance of cure or long-term survival for these patients. Almost 40% of patients with PDAC show locally advanced pancreatic cancer (LAPC). LAPC is not a metastatic disease, although it is not amenable to radical surgery.
For these patients, systemic induction chemotherapy with intravenous FOLFIRINOX (5-fluorouracil, folic acid, irinotecan, oxaliplatin) regimen is administered, with the aim of conversion to surgery, although the conversion rate remains low, at approximately 10% to 15%. Pancreatic arterial chemotherapy has been explored to overcome the intrinsic tumor pancreatic resistance to systemic chemotherapy, where an intra-arterial port-a-cath is placed by means of interventional oncology techniques under angiographic guidance in the operating theater. Here, we treated a patient with an intra-arterially modified FOLFIRINOX regimen.
Three courses were administered, and the patient experienced no adverse events. At the end of the third course, the patient rapidly developed lung failure due to nosocomial Legionella pneumophila infection, despite the impressive pathological tumor response shown in the autopsy report. This is a first and unique report that demonstrates that pancreatic intra-arterial FOLFIRINOX can be safe and efficacious. We believe that this preliminary result will be confirmed in the next patients to be enrolled and that it provides a glimmer of hope for patients with this lethal disease.
Introduction
Patients affected by pancreatic ductal adenocarcinoma (PDAC) have very poor prognosis, with the mortality rate very similar to the incidence rate at the follow-up of 5 years. Globally, around 10% of patients are amenable to radical surgery at the time of diagnosis, which represents the only chance of cure or long-term survival for these patients.
Almost 40% of patients with PDAC show locally advanced pancreatic cancer (LAPC) , and although this is not a metastatic disease, it is not amenable to radical surgery. This is due to T4 presentation that involves infiltration of the celiac artery, the superior celiac artery, and/or the venous splenic–portal–mesenteric axis, without or with regional lymph node involvement, indicative of stage III according to the TNM classification (8th edition) (4). For these patients, systemic induction chemotherapy with an intravenous (IV) FOLFIRINOX (5-fluorouracil, folic acid, irinotecan, oxaliplatin) regimen is administered, with the aim of conversion to surgery, although with a low conversion rate (10%–15%). This low rate is due to several biological and pathological characteristics, among which abundant desmoplastic tissue is a relevant barrier to chemotherapy penetration into the tissue microenvironment.
To overcome the low chemotherapy efficacy, several studies have explored the possibility to administer intra-arterial (IA) pancreatic infusion chemotherapy through interventional radiology techniques, in particular with gemcitabine, platinum salts, or 5-fluorouracil. These studies have obtained very interesting results in terms of safety, tolerability, and response rates, with low systemic toxicity. IA infusion chemotherapy has been mainly evaluated in liver colorectal cancer metastases and hepato-biliary cancers, and it is performed in specialized centers with interventional radiology and loco-regional oncological medical expertise in a multidisciplinary team organization.
The biological background of IA pancreatic infusion therapy is to realize high intra-tumoral concentrations of the chemotherapeutic agents with the longest intra-tumor times, for prolonged drug bioavailability and low systemic toxicity (19). To administer IA chemotherapy, a port-a-cath device is placed into the artery using interventional radiology techniques.
Case Presentation
First Hospitalization
A 68-year-old woman suffering from recurrent upper abdominal pain in the previous 3 months was hospitalized on December 14, 2020, at the Interventional and Medical Oncology Unit of the National Cancer Institute, Giovanni Paolo II of Bari (Bari, Italy). She had no history of smoking, alcohol consumption, diabetes, known chronic pancreatitis, obesity, or familial cancer. She had a 10-year history of rheumatoid arthritis that was initially treated with prednisone and methotrexate, and then in the previous 2 years, etanercept had been added to obtain better control of the rheumatic disease. For her recent history, she had not shown any vomiting, jaundice, weight loss, or loss of appetite.
Physical examination showed multiple, non-adherent, palpable lateral-cervical, and axillary lymph nodes (maximum diameter, 1 cm). Some swelling of the fingers and wrist joints was also seen. At the time of hospital admission, the patient had undergone a thorax-abdomen computed tomography (CT) scan (October 13, 2020) that had shown a hypodense nodule at the head of the pancreas, with the maximum diameter of 26 mm and an upper abdomen nuclear magnetic resonance (NMR) (October 29, 2020) that had confirmed the pancreatic lesion with a maximum diameter of 25 mm. She also showed carcinoma embryonic antigen and carbohydrate antigen in the normal range, with a performance status according to the European Cooperative Oncology Group (ECOG) of 0 to 1.
During this initial hospitalization, the patient underwent ultrasound endoscopy (December 15, 2020), which showed an irregular hypoechogenic head pancreatic nodule with maximum dimensions of 32 mm × 24 mm, and vascular encasement of the gastroduodenal artery and the whole of the superior mesenteric vein. A fine needle biopsy was performed with a gauge 19 needle. A positron emission tomography/CT scan (December 17, 2020) demonstrated a large area of deoxy-glucose uptake in the pancreas (standardized uptake value,. A total body CT scan (December 18, 2020) showed a lesion at the head of the pancreas with dimensions of 31 mm × 28 mm, with superior mesenteric vein involvement (Figure 1).
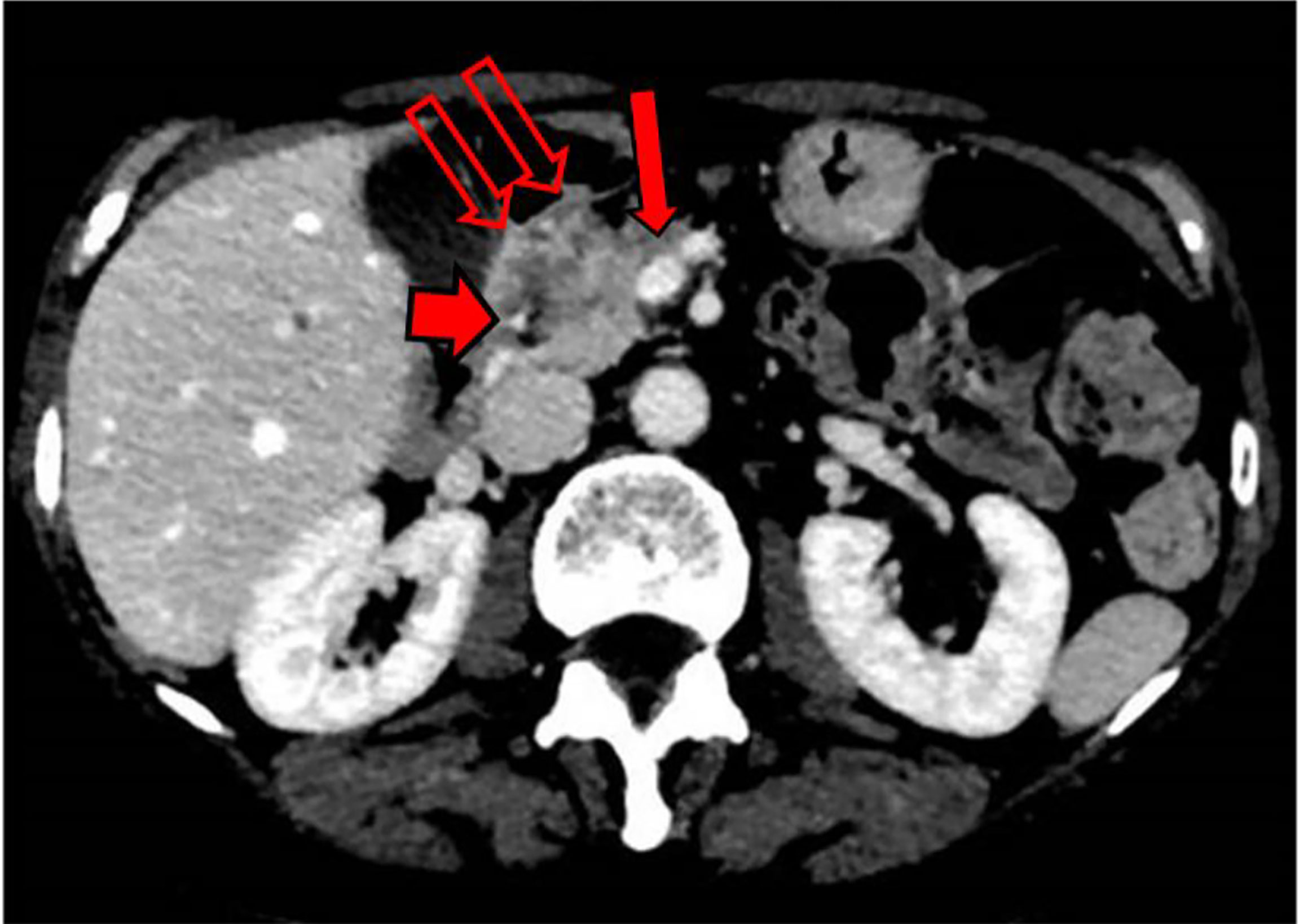
Further NMR imaging of the upper abdomen (December 22, 2020) confirmed a hypo-vascular tumor with dimensions of 30 mm × 30 mm for the head and the uncinate process of the pancreas. The tumor had infiltrated the superior mesenteric vein, which had resulted in reductions in the circumferences of the portal vein (>180°) and the superior mesenteric artery (<180°). A reduction in the circumference of the inferior vena cava was also seen, with no signs of vascular wall infiltration. Concomitant CA-19.9 assessment indicated an increased value of 163.8 U/ml.
The patient was discharged while awaiting the pathological diagnosis.
Second Hospitalization
The pathological diagnosis was complete in January 2021, and it revealed a PDAC associated with necrotic and inflammatory phenomena (Figure 2). Based on the clinical and pathological diagnoses, the patient was again hospitalized at the Interventional and Oncology Unit of the National Cancer Institute Giovanni Paolo II of Bari.
An updated total body CT scan (January 18, 2021) demonstrated the presence of the known tumor, with 30 mm × 30 mm maximum dimensions and a retro-dilated pancreatic Wirsung duct (diameter, 5 mm). The tumor had further infiltrated the superior mesenteric vein, which had resulted in increased reduction of the circumference (>180°) and also involved the portal vein.
Evaluation of all of the radiology examinations of the PDAC of the patient resulted in its staging as IIIB, according to the TNM classification (8th edition), which was not amenable to radical surgical resection. Due to the low efficacy of systemic FOLFIRINOX as an induction therapy, the patient was offered enrolment in a phase II experimental protocol ongoing at the Institute. The patient accepted the experimental therapy and provided written informed consent to participate in the study.
Experimental Clinical Study
This experimental clinical protocol was approved in 2020 by the local Ethical Committee of the National Cancer Institute Giovanni Paolo II of Bari (ID N° 948). The study was designed to treat patients with LAPC.
In summary, the study involves infusion of FOLFIRINOX via the pancreatic IA route, with the following modified schedule administered on day 1: oxaliplatin 85 mg/m2 over 2 h; leucovorin 400 mg/m2 over 2 h; irinotecan 130 mg/m2 over 90 min; and fluorouracil 2,400 mg/m2 as a continuous infusion over 46 h (i.e., starting on day 1). The use of bolus 5-fluorouracil administration was avoided in this schedule (28). Each cycle was for 14 days.
The infusions were carried out using an electromechanical pump to overcome the pressure of the arterial system. To avoid endothelial arterial damage, dexamethasone 8 mg was administered via the pancreatic artery before the start of the chemotherapy, and then after the infusion of 5-fluorouracil. Systemic premedication was administered IV with palonosetron 0.25 mg, chlorphenamine 10 mg, and omeprazole 40 mg.
The main inclusion trial criteria were as follows: pathological diagnosis of PDAC; inoperable stage III LAPC; arterial and/or venous vascular encasement by the tumor; ECOG performance status 0 to 1; age, 18 to 80 years; American Society of Anesthesiologists classification from 1 to 3; adequate hematological parameters (hemoglobin ≥9 g/dl; absolute neutrophil count ≥1,500/mm3; platelet count ≥100,000/mm3); prothrombin time with an international normal ratio ≤1.5 times the upper limit of normal; adequate renal function (serum creatinine, ≤1.5 times the upper limit of normal; creatinine clearance calculated by Cockroft–Gault formula, ≤30 ml/min); adequate hepatic function (aspartate amino transferase, aspartate alanine transferase levels, ≤2.5 times the upper limits of normal; bilirubin, ≤2.5 mg/dl); and written informed consent.
The main exclusion criteria were for metastatic disease, ascites, infected tumor, other previous or concomitant malignant tumors, pregnancy, high risk for no cardiac surgery, presence of metallic stent, HIV, HBV, and HCV infection.
As previously described, the patient was enrolled into this protocol.
Interventional Technical Procedure
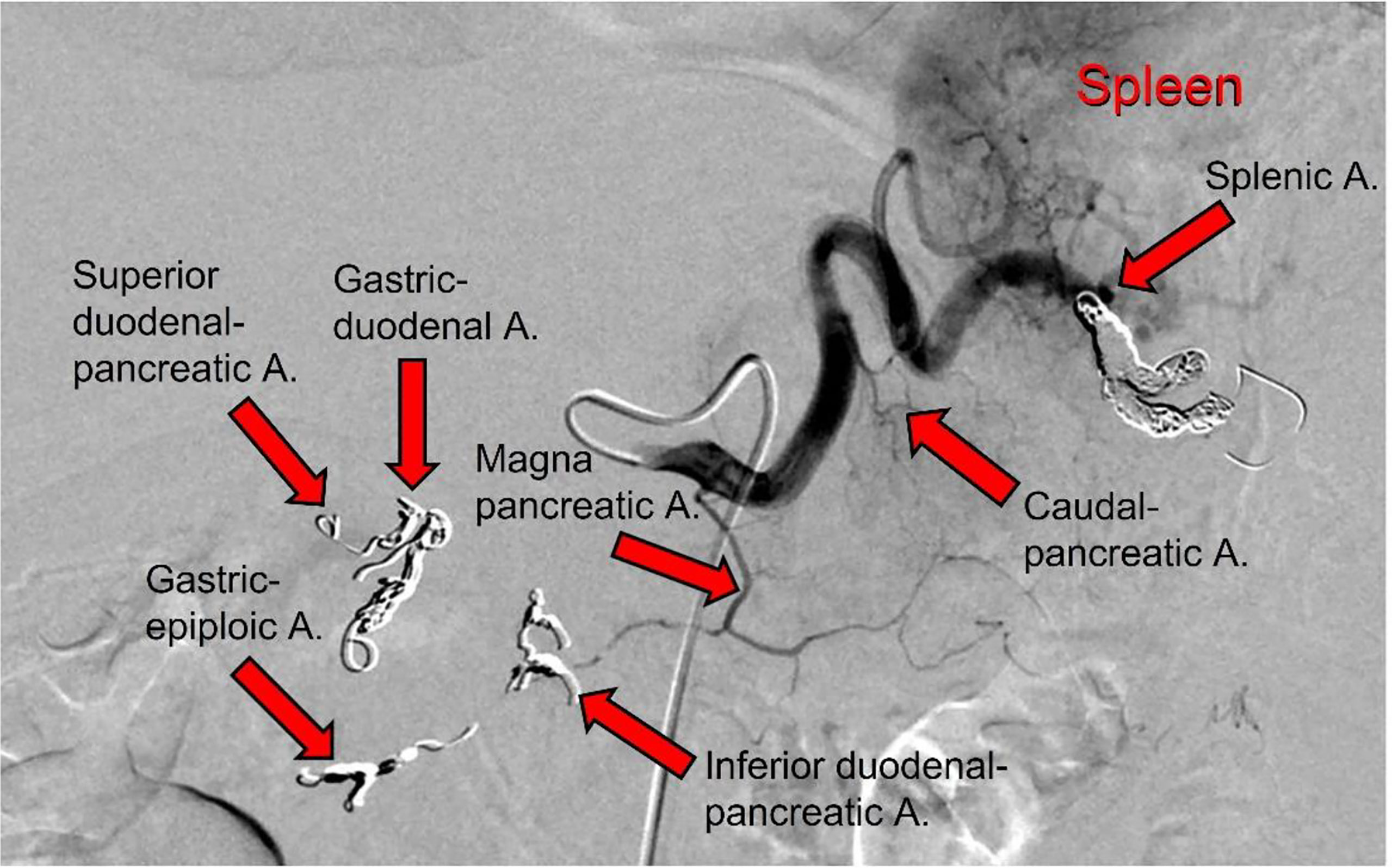
During this second hospitalization period for the patient (January 19, 2021), an IA port-a-cath device was implanted. Briefly, in the angio-CT operating theater, under general anesthesia, and using ultrasonic guidance, avascular sheath (6 Fr; Introducer II Standard Kit A; Terumo Radifocus) was inserted into the right femoral artery; this was passed into the aorta artery and then introduced into the celiac axis. Next, microcatheters (2.7/2.4-Fr coaxial microcatheter system; Terumo Progreat) were introduced into the gastroduodenal artery, respectively superior mesenteric artery and splenic artery. All arterial rami were non-pancreatic directed, and the superior and inferior pancreatic duodenal arteries were embolized using magnetic spiral devices (Helix 3D detachable coil system; Axium). This vascular modification was performed to avoid the escape of blood from the pancreas, and in this way the pancreatic arterial system was all sustained by the splenic artery and the ramus from which it originated, including the main great pancreatic artery, which together with the caudal pancreatic artery was anastomosed with the transversal pancreatic artery. Subsequently, the tip of the hydrophilic diagnostic catheter (4 Fr; Glidecath angiographic catheter; Radifocus) was placed at the level of the splenic hilum, and then the hydrophilic guide was inserted into this, to allow the hydrophilic diagnostic catheter to be removed and the definitive placement of the polyurethane catheter (6.5 Fr; PolyFlow polyurethane single-lumen portal; Smiths Medical, Minneapolis, MN, USA) for the IA FOLFIRINOX infusion. At the end of the technical procedure, a selective splenic arterial examination was performed (Figure 3).
 Patient Treatment
Patient Treatment
First Cycle of IA FOLFIRINOX Infusion
After the interventional procedure, the patient was monitored for several days, with no complications or adverse effects observed. The first cycle of IA FOLFIRINOX was then started on January 26, 2021.
Immediately before starting this IA therapy, the trans-port-a-cath angiography demonstrated the patency of the remodeled pancreatic arterial system. Then, the IA FOLFIRINOX was administered and was seen to be very well tolerated, without side effects and without any hematological grade toxicity.
The patient was discharged from the hospital.
Second Cycle of IA FOLFIRINOX Infusion, as an Outpatient
The second cycle was administered on February 9, 2021, and again before the therapy, the trans-port-a-cath angiography demonstrated the patency of the pancreatic arterial system. The ECOG performance status of the patient remained at 0 to 1.
Over the following 2weeks, the patient felt good and showed no clinical side effects or hematological toxicity.
Third and Last Hospitalization
The trans-port-a-cath angiography before the third cycle of the therapy on February 24, 2021, showed thrombosis of the great pancreatic artery. Due to this complication, the patient was hospitalized at the Interventional and Medical Oncology Unit of the National Cancer Institute, Giovanni Paolo II of Bari. She was immediately administered trans-port-a-cat artery thrombolytics, with continuous infusions of urokinase and both IA and IV dexamethasone. The urokinase and corticosteroid infusions were continued, and on February 27, 2021, the trans-port-a-cath angiography indicated partial resolution of the pancreatic artery thrombus. The urokinase and corticosteroid infusions were prolonged, until they were stopped on March 1, 2021, when the trans-port-a-cath angiography indicated the disappearance of the arterial thrombus.
CA-19.9 assessment (March 3, 2021) showed a value of 543.4 U/ml. CT scan evaluation (March 8, 2021) demonstrated a partial response according to the RECIST evaluation criteria (20). This was confirmed by NMR abdomen examination (March 10, 2021), with ECOG performance status well maintained (0-1). On the same day, the trans-port-a-cath angiography confirmed the patency of the arterial pancreatic system, and the third cycle of IA FOLFIRINOX was started.
Unexpectedly, the patient complained of malaise on March 11, 2021, with tachypnea and fast heartbeat. Diagnosis of paroxysmal atrial fibrillation was made, and regression was obtained within 2 h through IV infusion of amiodarone. In addition, on pulmonary auscultation, snores and gasps were evident. Blood gas analysis showed a very low oxygen pressure of 47.7 mmHg. A thorax CT scan demonstrated bilateral pleural effusion, bilateral pulmonary thickening, and ground-glass areas with initial aspects of parenchymal consolidation. Based on radiological findings, the diagnosis of bilateral pneumonia of probable bacterial etiology was made. Oxygen therapy (3 l/min) was added. After infectious disease consultation, broad-spectrum antibiotic therapy was started with piperacillin plus tazobactam 4.5 g IV three times a day, clarithromycin 500 μg IV two times a day, sulfamethoxazole plus trimethoprim 1,920 mg IV three times a day, and ganciclovir 280 mg IV two times a day. Blood cultures and urinary antigenemia for Pneumococcus and Legionella pneumophila were performed. The patient also underwent bronchoscopy, and the broncho-alveolar lavage fluid obtained was used to investigate cytomegalovirus, SARS-CoV-2 coronavirus, pneumocystis carinii, and common bacteria and fungi.
The result of the urinary Legionella pneumophila antigenemia was available on March 12, 2021, at 3:00 p.m., and it was positive. At the same time, the serum search for IgG and IgM toward Legionella showed values of 1 (negative) and 40 (positive), respectively. The report of the infectious disease was forwarded to the Italian Health Authorities. Over the day, the patient showed hyperthermia of 38°C.
The continuous IA infusion of 5-fluorouracil was stopped on March 13, 2021. CA-19.9 assessment showed a lower value of 323 U/ml. During the day the patient worsened and showed marked asthenia and dyspnea. Blood gas analysis showed an oxygen pressure of 57.3 mmHg, and oxygen therapy (6 l/min) was applied using a mask (Venturi) over several hours. This provided increased blood oxygen pressure from 80 to 85 mmHg. After resuscitation consultation, continuous positive airway pressure ventilation replaced the oxygen therapy by mask, which obtained an improved blood oxygen pressure of 92 mmHg. In the late evening, the patient worsened again, and blood gas analysis showed a severe hypoxemic blood condition and acute respiratory acidosis.
During the night (March 14, 2021, 12:05 a.m.), the patient was transferred to the post-operatory Intensive Therapy Unit at the National Cancer Institute, Giovanni Paolo II of Bari. The patient was intubated, but at 01:30 a.m., the patient died due to irreversible respiratory failure.
Autopsy Report
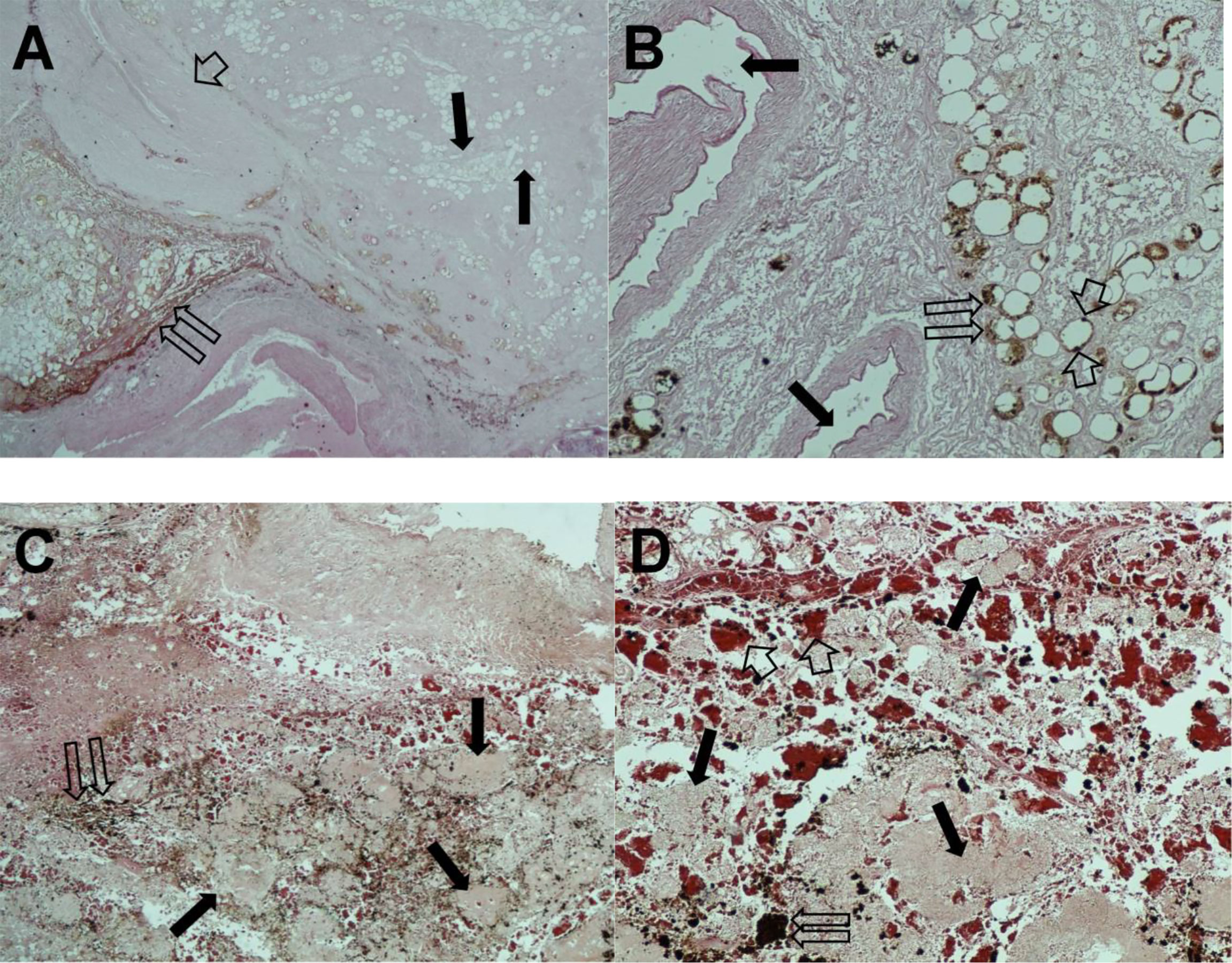
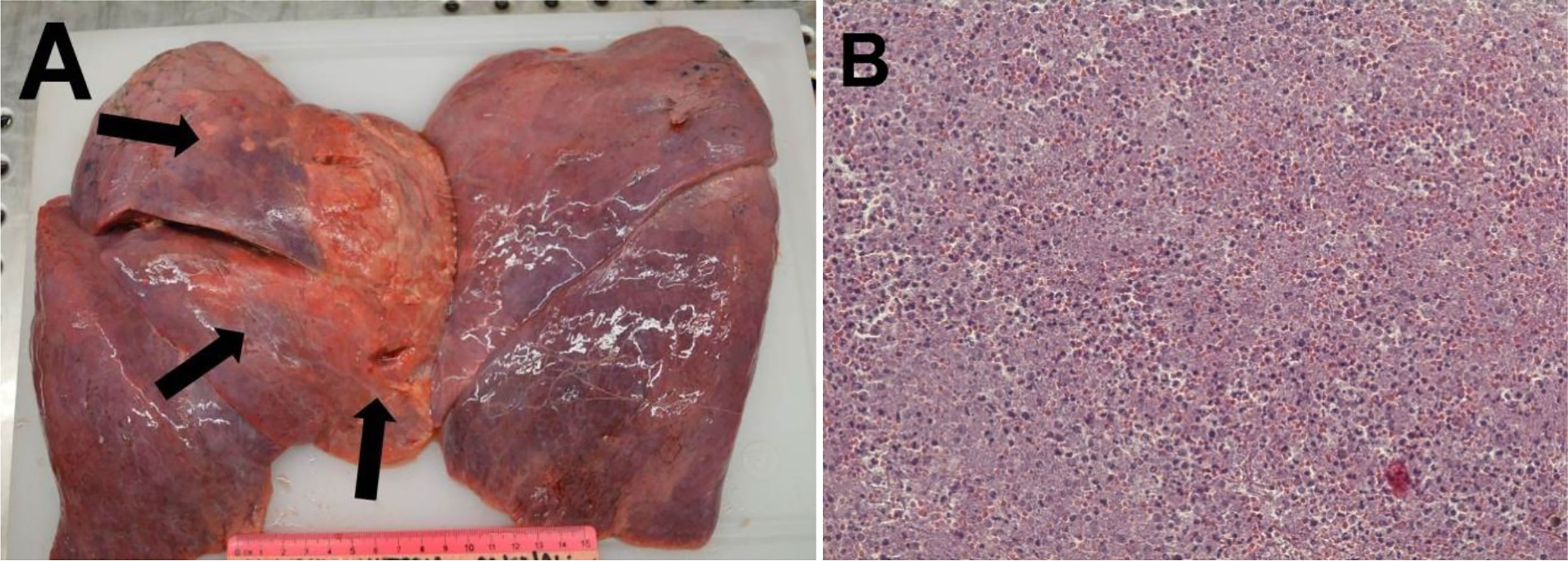
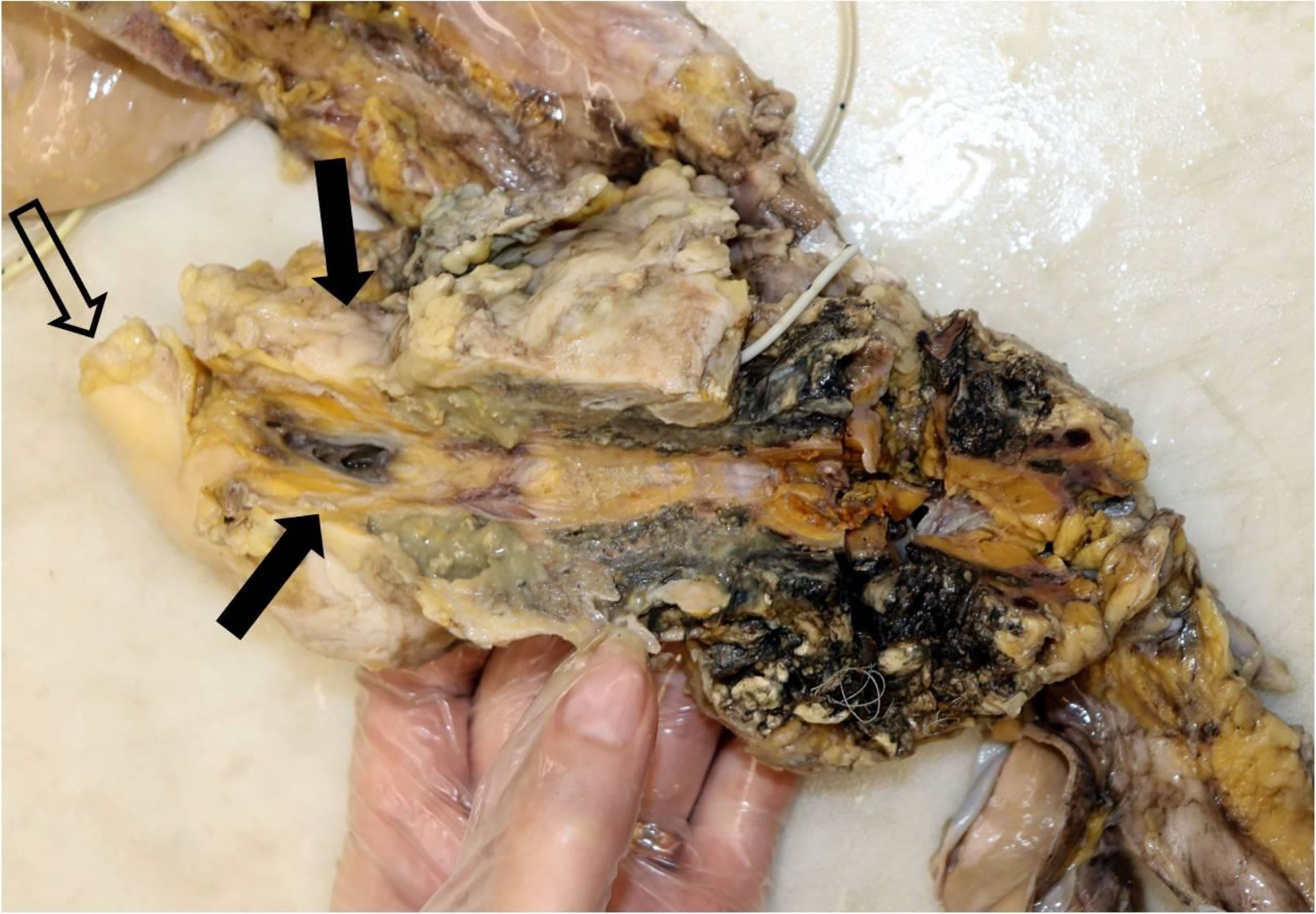
In the subsequent autopsy, the macroscopic pancreas observation indicated head volume reduction with complete tumor mass disappearance (Figure 4).
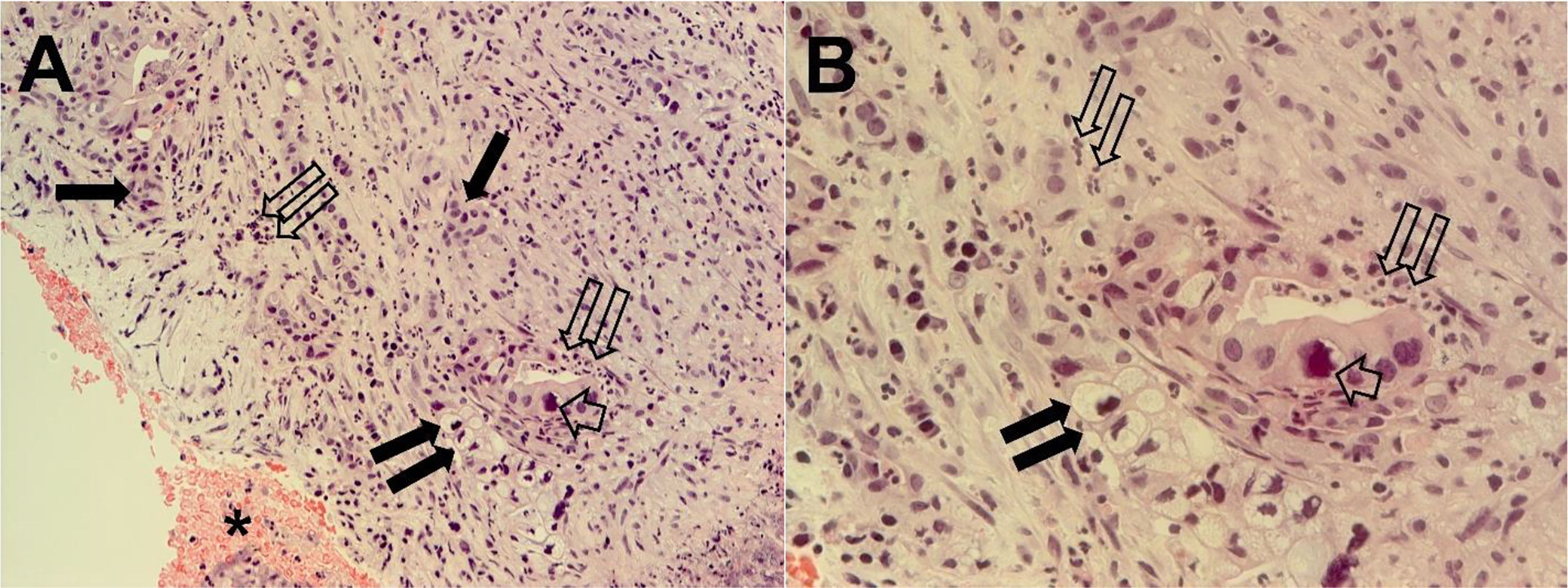
Histopathological examination of the pancreatic tissue showed complete pathological response for the PDAC, with extensive regressive phenomena of the malignancy associated with an interstitial fibrosclerosis reaction circumscribing the regressed tumoral tissue (Figure 5). Here, it is very important to underline that the complete histopathological evaluation of the non-cancerous pancreatic tissue did not show any signs of hemorrhagic–necrotic pancreatitis, which demonstrated that the IA chemotherapy had been safe and that it was not a factor in the induction of pancreatitis. With specific reference to the lung examination, there was a yellow-white color and an increased consistency at palpation particularly on the parietal surface of the right upper lobe (Figure 6A). Finally, the pulmonary level showed clear histopathological changes due to severe bacterial Legionella pneumophila infection (Figure 6B).