Neuroblastoma - Childhood - Introduction
ON THIS PAGE: You will find some basic information about this disease and the parts of the body it may affect. This is the first page of Cancer.Net’s Guide to Childhood Neuroblastoma. Use the menu to see other pages. Think of that menu as a roadmap for this entire guide.
Cancer begins when healthy cells change and grow out of control, forming a mass called a tumor. A tumor can be cancerous or benign. A cancerous tumor is malignant, meaning it can grow and spread to other parts of the body. A benign tumor means the tumor can grow but will not spread.
About neuroblastoma
Neuroblastoma is a solid cancerous tumor that begins most often in the nerve cells outside the brain of infants and children younger than 5. It can form in a baby before birth and can sometimes be found during a prenatal (before birth) ultrasound. More rarely, neuroblastoma can also develop in older children, adolescents, and young adults.
The most common place for neuroblastoma to begin is the adrenal glands, which are located on top of both kidneys. These glands make hormones that help control body functions, such as heart rate and blood pressure. Neuroblastoma can also start in the nerve tissue near the spine in the neck, chest, abdomen, or pelvis.
Neuroblasts are immature nerve cells found in an embryo, also called a fetus or unborn baby. Normally, neuroblasts mature into nerve cells or adrenal medulla cells, which are cells found in the center of the adrenal gland. Neuroblastoma forms when neuroblasts don’t mature properly.
Sometimes, babies are born with small clusters of neuroblasts that eventually mature into nerve cells and do not become cancer. But a neuroblast that does not mature normally can continue to grow, forming a tumor.
Neuroblastoma is usually found after the cancer has spread to other parts of the body, such as the lymph nodes (small, bean-shaped organs that help fight infection), liver, lungs, bones, and bone marrow (spongy, red tissue in the inner part of large bones.)
Looking for More of an Introduction?
If you would like more of an introduction, explore this related item::
The next section in this guide is Statistics . It helps explain the number of people who are diagnosed with neuroblastoma and general survival rates. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Statistics
ON THIS PAGE: You will find information about the estimated number of children who will be diagnosed with neuroblastoma each year. You will also read general information on surviving the disease. Remember, survival rates depend on several factors, and no 2 people with a tumor are the same. Use the menu to see other pages.
Every person is different, with different factors influencing their risk of being diagnosed with this tumor and the chance of recovery after a diagnosis. It is important to talk with your doctor about any questions you have around the general statistics provided below and what they may mean for your child individually. The original sources for these statistics are provided at the bottom of this page.
How many children are diagnosed with neuroblastoma?
Each year, about 700 to 800 children in the United States are diagnosed with neuroblastoma. Neuroblastoma accounts for 6% of all childhood cancers in the United States.
About 90% of neuroblastoma is found in children younger than 5. The average age of diagnosis is between 1 and 2 years old. Neuroblastoma is the most common cancer diagnosed in children younger than 1. It is rare in people older than 10.
What is the survival rate for neuroblastoma?
There are different types of statistics that can help doctors evaluate a person’s chance of recovery from neuroblastoma. These are called survival statistics. A specific type of survival statistic is called the relative survival rate. It is often used to predict how having a tumor may affect life expectancy. Relative survival rate looks at how likely people with neuroblastoma are to survive for a certain amount of time after their initial diagnosis or start of treatment compared to the expected survival of similar people without this tumor.
Example: Here is an example to help explain what a relative survival rate means. Please note this is only an example and not specific to this type of cancer. Let’s assume that the 5-year relative survival rate for a specific type of cancer is 90%. “Percent” means how many out of 100. Imagine there are 1,000 people without cancer, and based on their age and other characteristics, you expect 900 of the 1,000 to be alive in 5 years. Also imagine there are another 1,000 people similar in age and other characteristics as the first 1,000, but they all have the specific type of cancer that has a 5-year survival rate of 90%. This means it is expected that 810 of the people with the specific cancer (90% of 900) will be alive in 5 years.
It is important to remember that statistics on the survival rates for children with neuroblastoma are only an estimate. They cannot tell an individual person if the tumor will or will not shorten their life. Instead, these statistics describe trends in groups of people previously diagnosed with the same disease, including specific stages of the disease.
The 5-year relative survival rate for neuroblastoma in children under age 15 is 82%.
The survival rates for neuroblastoma vary based on several factors. These include the stage and risk grouping of the tumor, a person’s age and general health, and how well the treatment plan works.
For children with low-risk neuroblastoma, the 5-year relative survival rate is higher than 95%. For children with intermediate-risk neuroblastoma, the 5-year relative survival rate is between 90% and 95%. For children with high-risk neuroblastoma, the 5-year relative survival rate is around 50%.
Experts measure relative survival rate statistics for neuroblastoma every 5 years. This means the estimate may not reflect the results of advancements in how neuroblastoma is diagnosed or treated from the last 5 years. Talk with your child’s doctor if you have any questions about this information. Learn more about understanding statistics .
Statistics adapted from the American Cancer Society website. Additional source was : Seigel R, et al.: Cancer Statistics 2023 . CA: A Cancer Journal for Clinicians . 2023 Jan; 73(1):17–48. doi/full/10.3322/caac.21763 . (All sources accessed February 2023.)
The next section in this guide is Medical Illustrations. It offers drawings of body parts often affected by neuroblastoma. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Medical Illustrations
ON THIS PAGE: You will find a drawing of the main body parts affected by childhood neuroblastoma. Use the menu to see other pages.
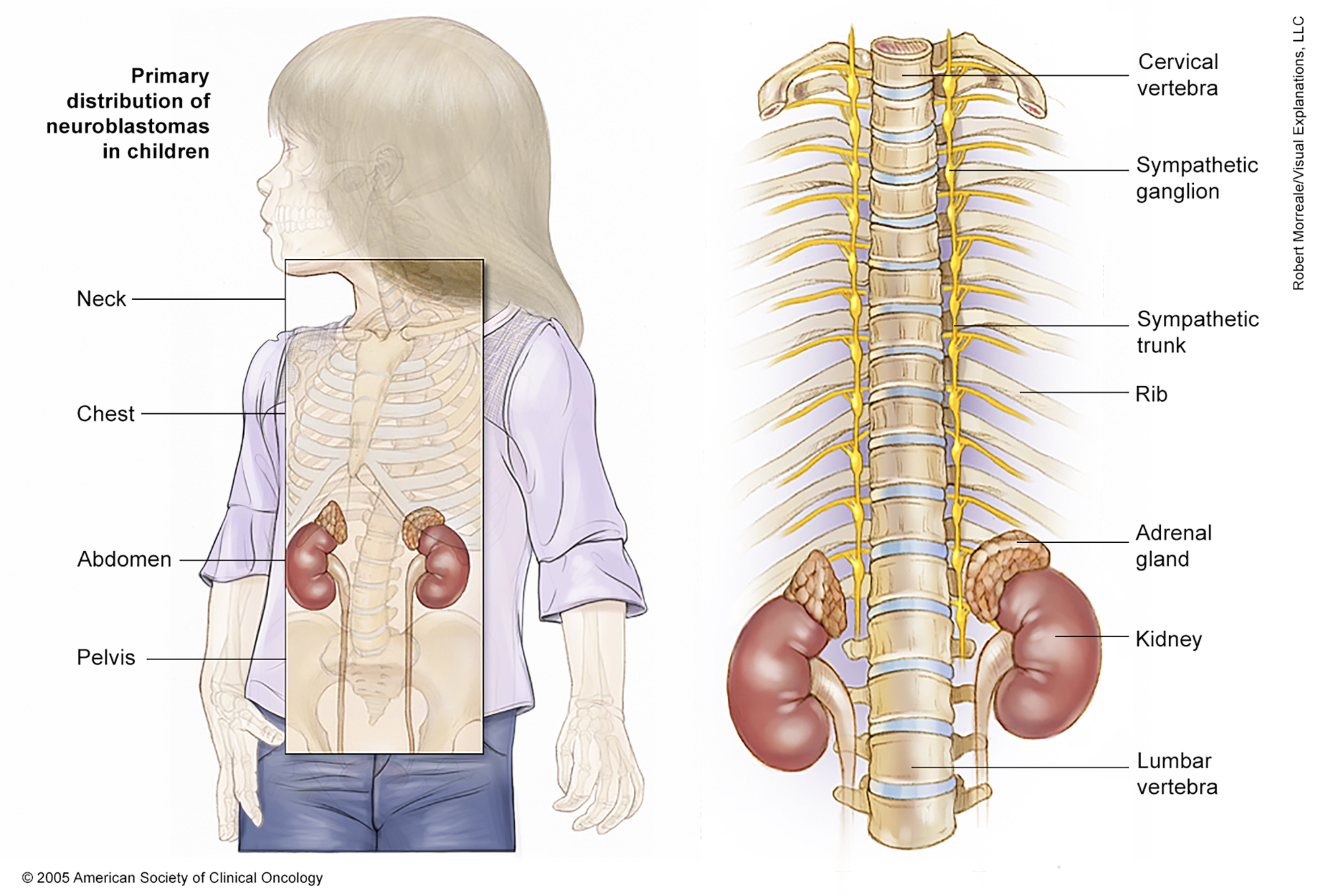
The next section in this guide is Risk Factors . It describes the factors that may increase the chance of developing childhood neuroblastoma. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Risk Factors
ON THIS PAGE: You will find out more about the factors that increase the chance of developing neuroblastoma. Use the menu to see other pages.
For most types of cancer, a “risk factor” is anything that increases a person’s chance of developing cancer. Although risk factors can influence the development of cancer, most do not directly cause cancer. Some people with several risk factors never develop cancer, while others with no known risk factors do.
For neuroblastoma, the term “risk factor” is more commonly used once a tumor has been found to describe the factors that are used to predict how the tumor will grow and how well treatment will work. Learn more about a tumor's risk grouping in this guide's section on Stages and Groups .
It is known that neuroblastoma occurs more often in boys than in girls. So far, no environmental factors have been shown to increase the risk of developing neuroblastoma.
Rarely, more than 1 member of a family is diagnosed with neuroblastoma. Researchers have found inherited gene mutations, or changes, that play a role in the development of neuroblastoma for children with a family history of the disease. Other genetic changes, called single-nucleotide polymorphisms (SNPs), may contribute to the development of neuroblastoma in children who do not have a family history.
Family history and genetic predisposition
Approximately 1% to 2% of children with neuroblastoma have a family history of the disease. Children with an inherited likelihood of neuroblastoma tend to develop the disease, on average, 9 to 13 months earlier than other children with neuroblastoma. In children who have a family history of neuroblastoma, the disease may occur in 2 or more organs.
Neuroblastoma has been diagnosed in people with congenital central hypoventilation syndrome (CCHS), a unique disorder of breathing control associated with Hirschsprung disease (HSCR). CCHS can result from germline mutations, which is a hereditary genetic mutation that is passed down from parent to child, or it may be a new genetic mutation in the child in the paired-like homeobox (PHOX) 2B gene. Mutations in the PHOX2B gene have been found in people with a family history of neuroblastoma and in most cases are associated with HSCR and CCHS. PHOX2B mutations have also been detected occasionally (less than 2% of cases) in DNA samples from neuroblastoma tumors in people with no family history of this disease.
In most people with a family history of neuroblastoma, there are germline mutations in the anaplastic lymphoma kinase (ALK) gene. These genetic mutations result in abnormal ALK activation. Activating ALK mutations have also been identified in DNA from neuroblastoma tumors in children without a family history, and in a subset of patients, ALK amplification is found in neuroblastoma tumors.
Neuroblastoma has also been diagnosed in several people who are missing portions of chromosomes 1p and 11q; these chromosomes are thought to prevent tumor growth.
Genetic factors without a family history
The genetic factors involved in development of neuroblastoma in patients with no family history of the disease are not yet well understood. Although a number of genes with germline mutations that raise the risk of developing neuroblastoma have been identified, including TP53 , NRAS, and BRCA2 , it is unclear what role they play. Common polymorphisms (including BARD1 or LMO1 ) have also been identified that have relatively small effects on the risk of a person developing neuroblastoma. In some cases, several polymorphisms can cooperate, leading to a higher risk of neuroblastoma. Research to identify more rare predisposition polymorphisms is ongoing.
Read more about the genetics of cancer . You can also learn more about this topic at the National Institute of Health’s website for the National Human Genome Research Institute (please note this link takes you to a separate website).
The next section in this guide is Symptoms and Signs . It explains what changes or medical problems childhood neuroblastoma can cause. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Symptoms and Signs
ON THIS PAGE: You will find out more about changes and other things that can signal a problem that may need medical care. Use the menu to see other pages.
Children with neuroblastoma may experience the following symptoms or signs. Symptoms are changes that you can feel in your body. Signs are changes in something measured, like by taking your blood pressure or doing a lab test. Together, symptoms and signs can help describe a medical problem. Sometimes, children with neuroblastoma do not have any of the symptoms and signs described below. Or, the cause of a symptom or sign may be a medical condition that is not cancer.
Many symptoms of neuroblastoma are caused by pressure from the tumor or bone pain if the cancer has spread to the bones. Bone pain may cause the child to limp, stop walking, or become unable to walk. Other symptoms may include:
-
A lump or mass in the abdomen, chest, neck, or pelvis
-
Skin lesions or nodules under the skin with blue or purple patches
-
Eyes that bulge out and dark circles under the eyes, if the cancer has spread behind the eyes
-
Changes in the eyes, such as black eyes, a droopy eyelid, a pupil that is constricted, vision problems, or changes in the color of the iris
-
Pain in the chest, difficulty breathing, or a persistent cough
-
Pain in the arms, legs, or other bones
-
Pain in the back, or weakness, numbness, or paralysis of the legs if the tumor has spread to the spinal cord
-
Changes in bowel or bladder function
-
Fever and anemia, which is a low level of red blood cells
-
Constant diarrhea or high blood pressure caused by hormones released by the tumor
-
Rotating movements of the eyes and sudden muscle jerks, likely from immune system problems caused by the disease
If you are concerned about any changes your child experiences, please talk with your child’s doctor. Your doctor will ask how long and how often your child has been experiencing the symptom(s), in addition to other questions. This is to help figure out the cause of the problem, called a diagnosis.
If neuroblastoma is diagnosed, relieving symptoms remains an important part of care and treatment. Managing symptoms may also be called "palliative care" or "supportive care." It is often started soon after diagnosis and continued throughout treatment. Be sure to talk with the health care team about the symptoms your child experiences, including any new symptoms or a change in symptoms.
The next section in this guide is Diagnosis . It explains what tests may be needed to learn more about the cause of the symptoms. Use the menu to choose a different section to read this guide.
Neuroblastoma - Childhood - Diagnosis
ON THIS PAGE: You will find a list of the common tests, procedures, and scans that doctors can use to find out what’s wrong and identify the cause of the problem. Use the menu to see other pages.
Doctors use many tests to find, or diagnose, a tumor. They also do tests to learn if cancer has spread to another part of the body from where it started. If the cancer has spread, it is called metastasis. Doctors may also do tests to learn which treatments could work best.
For most tumors, a biopsy is the only sure way for the doctor to know if an area of the body has cancer. In a biopsy, the doctor takes a small sample of tissue for testing in a laboratory. If a biopsy is not possible, the doctor may suggest other tests that will help make a diagnosis.
How neuroblastoma is diagnosed
There are many tests used for diagnosing neuroblastoma. Your child’s doctor may consider these factors when choosing a diagnostic test:
-
The type of cancer suspected
-
Your child’s signs and symptoms
-
Your child’s age and general health
-
The results of earlier medical tests
According to international criteria developed by an International Neuroblastoma Risk Group task force, a diagnosis of neuroblastoma can be made if:
-
Neuroblastoma cells are detected in the bone marrow and there are higher than normal levels of one of the main chemicals produced by the nervous system, called catecholamine, present in the urine
-
Results of a tumor biopsy show neuroblastoma cells
In addition to a physical examination, the following tests may be used. Not all tests described here will be used for every child. Talk with your doctor about tests your child may have.
-
Blood tests and urine tests.
-
Complete blood counts (CBC) are tests to find out if the child has signs of anemia, which is having low levels of red blood cells in the blood. Additional blood tests will be done to check liver and kidney function. In addition, blood clotting tests may also be recommended.
-
Urine is collected to test for tumor markers produced by a neuroblastoma tumor. A tumor marker is a substance found in higher than normal amounts in the blood, urine, or body tissues of people with certain kinds of cancer.
Urinary catecholamine metabolites are found in more the 85% of people with neuroblastoma. Catecholamines are organic compounds that include the hormones epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine. Release of the hormones epinephrine and norepinephrine from the adrenal medulla of the adrenal glands is part of the body's natural "fight-or-flight" response to a harmful situation, preparing itself to either quickly leave the area or stay to fight. Eventually, the body breaks down the catecholamine molecules into smaller pieces, called metabolites, which are then passed out of the body in the urine. The 2 catecholamine metabolites most often measured are homovanillic acid (HVA) or vanillylmandelic acid (VMA).
-
-
Biopsy. A biopsy is the removal of a small amount of tissue for examination under a microscope. A pathologist then analyzes the sample(s). A pathologist is a doctor who specializes in interpreting laboratory tests and evaluating cells, tissues, and organs to diagnose disease. The type of biopsy performed depends on the location of the tumor. If the surgeon determines that the entire tumor can be removed, the whole tumor is commonly removed instead of doing a separate biopsy.
-
Bone marrow aspiration and biopsy. These 2 procedures are similar and often done at the same time to examine the bone marrow. Bone marrow has both a solid and a liquid part. A bone marrow aspiration removes a sample of the fluid with a needle. A bone marrow biopsy is the removal of a small amount of solid tissue using a needle.
A pathologist then analyzes the samples. A common site for a bone marrow aspiration and biopsy is the pelvic bone, which is located in the lower back by the hip. In many centers, children are sedated before this procedure using a medication that a doctor will give called anesthesia to numb the area. Anesthesia is a medication that blocks the awareness of pain. Stronger types of anesthesia can also be used to lessen the pain.
-
Biomarker testing of the tumor/genetic tests. Your child's doctor may recommend running laboratory tests on the tumor sample to identify specific genes, proteins, and other factors unique to the tumor. Tests of neuroblastoma cell DNA are used to find a change in the oncogene MYCN , a gene responsible for cell growth. Having more than 10 copies of the gene, called amplification, is associated with a tumor that grows and spreads quickly, which makes it more difficult to treat. Having a non-amplified MYCN gene is linked to less aggressive tumors, which grow and spread more slowly.
Additional tests may be done to find out if the tumor has changes in the numbers of the whole chromosome or parts of chromosomes. A series of studies have shown that segmental chromosomal irregularities are associated with more aggressive disease, while whole chromosome gains are seen in tumors with more favorable outcomes. DNA sequencing tests to find out if there are mutations of the ALK gene are also commonly done. Sequencing studies have shown that a small subset of neuroblastoma tumors have gene mutations at the time of diagnosis, and some of these mutations, like ALK , can be targeted with new drugs. Studies have shown that tumors that come back, called recurrent tumors, often have increased gene mutation frequency.
For people with a family history of neuroblastoma (see Risk Factors ), genetic tests to determine whether the person has germline mutations in the PHOX2B or ALK genes are commonly done.
-
Computed tomography (CT or CAT) scan. A CT scan takes pictures of the inside of the body using x-rays taken from different angles. A computer combines these pictures into a detailed, 3-dimensional image that shows any abnormalities or tumors. A CT scan can be used to measure the tumor’s size. A special dye called a contrast medium is usually given before the scan to provide better detail on the image. This dye is injected into a patient’s vein or given as a pill or liquid to swallow.
-
Magnetic resonance imaging (MRI). An MRI uses magnetic fields, not x-rays, to produce detailed images of the brain and spinal column. MRI can be used to measure the tumor’s size. A special dye called a contrast medium is given before the scan to create a clearer picture. This dye can be injected into a patient’s vein or given as a pill or liquid to swallow. An MRI is better at showing neuroblastoma around the spine, and it is essential to look at a tumor that is near where nerves leave the spinal column, which can press on the spinal cord.
-
Meta-iodobenzylguanidine (MIBG) scan. MIBG is a protein that neuroblastoma cells absorb. When the protein is linked to a small amount of radioactive iodine, it can be used to find neuroblastoma in the bone, bone marrow, and other parts of the body. Because the child’s thyroid gland will also absorb radioactive iodine, regular iodine is taken by mouth for several days before the scan to protect the thyroid.
-
Positron emission tomography (PET) or PET-CT scan. A PET scan is usually combined with a CT scan (see above), called a PET-CT scan . However, you may hear your child's doctor refer to this procedure just as a PET scan. A PET scan is a way to create pictures of organs and tissues inside the body. A small amount of a radioactive substance is injected into a patient’s body. This substance is absorbed mainly by organs and tissues that use the most energy. Because cancer tends to use energy actively, it absorbs more of the radioactive substance. However, the amount of radiation in the substance is too low to be harmful. A scanner then detects this substance to produce images of the inside of the body. This test is usually performed when a patient's tumor does not absorb MIBG (see above).
After diagnostic tests are done, your child’s doctor will review the results with you. If the diagnosis is neuroblastoma, these results also help the doctor describe the cancer. This is called staging and risk grouping.
The next section in this guide is Stages and Groups . It explains the system doctors use to describe the extent of the disease. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Stages and Groups
ON THIS PAGE: You will learn about how doctors describe neuroblastoma’s growth or spread. This is called the stage. Use the menu to see other pages.
What is cancer staging?
Staging is a way of describing a cancer, such as where it is located, if or where it has spread, and whether it is affecting other parts of the body.
Doctors use diagnostic tests to find out the cancer's stage, so staging may not be complete until all of the tests are finished. Knowing the stage helps the doctor to recommend the best kind of treatment and can help predict a patient's prognosis, which is the chance of recovery. There are different stage descriptions for different types of cancer. Once a diagnosis of neuroblastoma is confirmed, how much the tumor has grown and spread is evaluated or staged.
The International Neuroblastoma Staging System Committee (INSS), a surgical/pathological staging system developed in 1988, was the first internationally accepted staging system for neuroblastoma. In 2009, the International Neuroblastoma Risk Group (INRG) Task Force proposed a new staging system (INRGSS) that was based on imaging. Each is described below, followed by an explanation of risk groupings.
-
The International Neuroblastoma Risk Group Staging System (INRGSS)
-
International Neuroblastoma Staging System Committee (INSS) system
-
Risk groups for neuroblastoma
-
Recurrent and refractory neuroblastoma
The International Neuroblastoma Risk Group Staging System (INRGSS)
The INRGSS was designed specifically for the International Neuroblastoma Risk Group (INRG) pre-treatment classification system (see "Risk groups," below). Unlike the INSS explained below, the INRGSS uses only the results of imaging tests taken before surgery. It does not include surgical results or spread to lymph nodes to determine the stage. Knowledge regarding the presence or absence of image-defined risk factors (IDRF) is required for this staging system.
Stage L1: The tumor is located only in the area where it started; no IDRFs are found on imaging scans, such as a computed tomography (CT) or magnetic resonance imaging (MRI) scan.
Stage L2: The tumor has not spread beyond the area where it started and the nearby tissue; IDRFs are found on imaging scans, such as a CT or MRI scan.
Stage M: The tumor has spread to other parts of the body (except stage MS; see below).
Stage MS: The tumor has spread to only the skin, liver, and/or bone marrow (with less than 10% bone marrow involvement) in a patient younger than 18 months.
Return to top
International Neuroblastoma Staging System Committee (INSS) system
This is the staging system previously used for neuroblastoma. The following is a brief summary of each INSS stage.
Stage 1: The tumor can be removed completely during surgery. Lymph nodes attached to the tumor removed during surgery may or may not contain cancer, but other lymph nodes near the tumor do not.
Stage 2A: The tumor is located only in the area it started and cannot be completely removed during surgery. Nearby lymph nodes do not contain cancer.
Stage 2B: The tumor is located only in the area where it started and may or may not be completely removed during surgery, but nearby lymph nodes do contain cancer.
Stage 3: The tumor cannot be removed with surgery. It has spread to regional lymph nodes (lymph nodes near the tumor) or other areas near the tumor, but not to other parts of the body.
Stage 4: The original tumor has spread to distant lymph nodes (lymph nodes in other parts of the body), bones, bone marrow, liver, skin, and/or other organs, except for those listed in stage 4S, below.
Stage 4S: The original tumor is located only where it started (as in stage 1, 2A, or 2B) and has spread only to the skin, liver, and/or bone marrow in infants younger than 1. The spread to the bone marrow is minimal (usually less than 10% of cells examined show cancer).
Return to top
Risk groups for neuroblastoma
Neuroblastoma grows and reacts differently to treatment in different people. This is called the disease’s clinical behavior. Some children are cured with surgery alone or surgery with chemotherapy (see Types of Treatment ). Others have a very aggressive disease that is resistant to treatment and difficult to cure. By using a combination of factors, doctors can usually predict how clinically aggressive the tumor will be and tailor treatments accordingly.
In the INRG classification system, a combination of clinical, pathologic, and genetic markers are used to predict the clinical behavior of the tumor and how it will respond to treatment. These markers are used to define risk. Each neuroblastoma is classified into 1 of 4 categories (very low-risk, low-risk, intermediate-risk, or high-risk) using the following factors:
-
The stage of the disease according to the INRGSS (see above)
-
The child's age at the time of diagnosis
-
Histologic category, such as maturing ganglioneuroma versus ganglioneuroblastoma, intermixed versus ganglioneuroblastoma, or nodular versus neuroblastoma
-
Grade, or how cells of the tumor are differentiated
-
MYCN gene status
-
Chromosome 11q status
-
Tumor cell ploidy, which is the DNA content of tumor cells
The Children’s Oncology Group currently uses the following factors to determine risk:
-
The stage of the disease according to the INRG system (see above)
-
The child's age at the time of diagnosis
-
MYCN gene status
-
Tumor ploidy (this is only important for children younger than 18 months)
-
Tumor histopathology according to the International Neuroblastoma Pathologic Classification (INPC) system
-
Segmental chromosomal aberration (SCA) status, or changes in the chromosome number or structure
Descriptions of low-risk, intermediate-risk, or high-risk neuroblastoma according to the current Children’s Oncology Group definitions are listed below.
Low-risk neuroblastoma
-
Stage L1 in infants younger than 1 year of age with a tumor smaller than 5 centimeters (cm) who have not undergone surgery and are observed, also called "wait and watch" (see Types of Treatment ). This type of neuroblastoma is also called "provisional low risk."
-
Stage L1 in infants and children with tumors that were completely removed with surgery (see Types of Treatment )
-
Stage L1 in infants and children with tumors with no MYCN amplification that have not been completely removed with surgery
-
Stage MS in infants younger than 1 year with no tumor-related symptoms, no MYCN amplification, and all favorable biology
Intermediate-risk neuroblastoma
-
Stage L2 with no MYCN amplification in children younger than 18 months with or without all favorable biology, children 18 months to 5 years old with favorable histology, and children 5 years or older with differentiating histology
-
Stage M in infants younger than 1 year with no MYCN amplification and children 1 year to 18 months with no MYCN amplification and all favorable biology
-
Stage MS in infants younger than 1 year with tumor-related symptoms, or with no tumor-related symptoms and no MYCN amplification but any unfavorable biology
-
Stage MS in children 1 year to 18 months with no MYCN amplification and all favorable biology
High-risk neuroblastoma
-
Stage L1 in infants younger than 1 year with a tumor 5 cm or larger in diameter that has not been completely removed with surgery and has MYCN amplification
-
Stage L1 in children 1 year or older that has not been completely removed with surgery and has MYCN amplification
-
Stage L2 with MYCN amplification
-
Stage L2 with no MYCN amplification in children 18 months to 5 years old with unfavorable histology or in children 5 years or older with undifferentiated or poorly differentiated histology
-
Stage M in children 18 months or older, or in children younger than 18 months with MYCN amplification
-
Stage M in children 1 year to 18 months with no MYCN amplification but any unfavorable biology
-
Stage MS in children younger than 1 year with MYCN amplification
-
Stage MS in children 1 year to 18 months either with MYCN amplification or with no MYCN amplification but any unfavorable biology
Descriptions of very low-risk, low-risk, intermediate-risk, or high-risk neuroblastoma according to INRG definitions are listed below.
Very low-risk neuroblastoma
-
Stage L1/L2 with ganglioneuroma maturing or ganglioneuroblastoma intermixed histology
-
Stage L1 with non-amplified MYCN
-
Stage MS in children younger than 18 months of age with no 11q aberration
Low-risk neuroblastoma
-
Stage L2 in children younger than 18 months of age with no 11q aberration
-
Stage L2 in children older than 18 months of age with ganglioneuroblastoma nodular or neuroblastoma with differentiating histology and no 11q aberration
-
Stage M in children younger than 18 months without MYCN amplification and hyperdiploidy
Intermediate-risk neuroblastoma
-
Stage L2 in children younger than 18 months without MYCN amplification with 11q aberration
-
Stage L2 in children older than 18 months with ganglioneuroblastoma nodular or neuroblastoma with differentiating histology with 11q aberration
-
Stage L2 in children older than 18 months with ganglioneuroblastoma nodular or neuroblastoma with poorly differentiated or undifferentiated histology
-
Stage M in children younger than 12 months with diploidy
-
Stage M in children 12 months to 18 months with diploidy
High-risk neuroblastoma
-
Stage L1 with MYCN amplification
-
Stage L2 with MYCN amplification
-
Stage M in children younger than 18 months of age with MYCN amplification
-
Stage M in children older than 18 months
-
Stage MS in children younger than 18 months with 11q aberration
-
Stage MS in children younger than 18 months of age with MYCN amplification
Treatment is tailored according to the risk assignment. Most patients with very low-risk and low-risk disease commonly receive surgery alone. Sometimes, infants with a small localized tumor have been successfully watched closely without any surgery, an approach called "observation" or "wait and watch."
Patients with intermediate-risk disease receive surgery and chemotherapy. In the Children’s Oncology Group, the most recently completed clinical trial for the intermediate-risk group found that the number of cycles of chemotherapy was determined by the presence or absence of tumor genetic changes in chromosomes 1p and 11q, tumor histology, tumor ploidy, stage, and the child's age.
A very intensive approach, often using several types of treatments, is used for patients with high-risk neuroblastoma. More information about specific treatment options can be found in the Types of Treatment section.
Return to top
Recurrent and refractory neuroblastoma
Recurrent
: Recurrent neuroblastoma is cancer that has come back after treatment. This is also called relapsed neuroblastoma. If cancer does return, there will be another round of tests to learn about the extent of the recurrence. These tests and scans are often similar to those done at the time of the original diagnosis.
Refractory:
If the cancer does not shrink or respond to treatment, it is called refractory neuroblastoma.
Return to top
Information about the cancer’s stage and risk group will help the doctor recommend a specific treatment plan. The next section in this guide is Types of Treatment . Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Types of Treatment
ON THIS PAGE: You will learn about the different treatments doctors use for children with neuroblastoma. Use the menu to see other pages.
In general, cancer in children is uncommon. This means it can be hard for doctors to plan treatments unless they know what has been most effective in other children. A clinical trial is a research study that tests a new approach to treatment. The “standard of care” is the best treatments known based on previous clinical trial results. Clinical trials may test such approaches as a new drug, a new combination of existing treatments, or new doses of current therapies. The health and safety of all children participating in clinical trials are closely monitored.
To take advantage of these newer treatments, children with cancer should be treated at a specialized cancer center. Doctors at these centers have extensive experience in treating children with cancer and have access to the latest research. A doctor who specializes in treating children with cancer is called a pediatric oncologist. If a pediatric cancer center is not nearby, general cancer centers sometimes have pediatric specialists who are able to be part of your child’s care.
How neuroblastoma is treated
In many cases, a team of doctors works with a child and the family to provide care. This is called a multidisciplinary team . Pediatric cancer centers often have extra support services for children and their families, such as child life specialists, dietitians, physical and occupational therapists, social workers, and counselors. Special activities and programs to help your child and family cope may also be available.
Treatment options and recommendations for neuroblastoma depend on several factors, including:
-
The size and location of the tumor
-
Whether the cancer has spread
-
The risk classification of the tumor
-
Possible side effects of treatment
-
Family preferences
-
The child’s overall health
In particular, the treatment plan is tailored based on the tumor's assigned risk group.
Take time to learn about all of your child’s treatment options and be sure to ask questions about things that are unclear. Talk with your child’s doctor about the goals of each treatment and what your child can expect while receiving the treatment. These types of talks are called “shared decision-making.” Shared decision-making is when you and your doctors work together to choose treatments that fit the goals of your child’s care. Shared decision-making is particularly important for childhood neuroblastoma because there are different treatment options. Learn more about making treatment decisions .
Descriptions of the most common treatment options for neuroblastoma are described below. Your child’s care plan also includes treatment for symptoms and side effects, an important part of cancer care.
-
Observation/wait and watch
-
Surgery
-
Chemotherapy
-
Radiation therapy
-
Stem cell transplantation/bone marrow transplantation
-
Immunotherapy
-
Retinoid therapy
-
Targeted therapy
-
Targeted delivery of radionuclides
-
Physical, emotional, and social effects of cancer
-
Remission and the chance of recurrence
-
If treatment does not work
Observation/wait and watch
A small group of infants with localized neuroblastoma do not require any active treatment, including surgery. Instead, the health of these patients is monitored closely by their doctors, an approach called "observation" or "wait and watch."
A previous Children’s Oncology Group study has demonstrated that it is safe to closely observe some infants younger than 6 months of age with a small tumor using imaging scans, physical exams, and laboratory tests. Over time, the majority of these neuroblastoma tumors regressed without further treatment. If there was evidence of tumor growth, the infants were then treated with surgery with or without chemotherapy, and all the patients enrolled on this clinical trial survived. In an ongoing Children's Oncology Group study, a wait and watch approach is being investigated for a broader population of patients that include those with International Neuroblastoma Risk Group staging system (INRGSS; see
Stages and Groups
) stage L1 tumors who are younger than 12 months of age at diagnosis with a tumor smaller than 5 centimeters located in adrenal or non-adrenal areas of the body.
Return to top
Surgery
Surgery is the removal of the tumor and some surrounding healthy tissue during an operation. A surgical oncologist is a doctor who specializes in treating cancer using surgery. If the cancer has not spread, surgery can sometimes be used to remove the entire tumor. However, most neuroblastoma is not found until after the cancer has spread. In that situation, the doctor can remove as much of the tumor as possible during surgery.
Even if a tumor cannot be removed because of its location, a surgical biopsy (see Diagnosis ) may still be done to determine the type of tumor.
If the tumor cannot be completely removed with surgery, the child may receive radiation therapy and chemotherapy (see below) after surgery to destroy the remaining cancer cells. In addition, the doctor may take a biopsy of the liver to find out if the disease has spread to the liver.
Talk with your child’s surgeon about the possible side effects of the surgery and how they will be relieved or managed. Learn more about the basics of cancer surgery .
Return to top
Chemotherapy
Chemotherapy is the use of drugs to destroy cancer cells, usually by keeping the cancer cells from growing, dividing, and making more cells. Chemotherapy is given by a pediatric oncologist or a medical oncologist, a doctor who specializes in treating cancer with medication.
Systemic chemotherapy gets into the bloodstream to reach cancer cells throughout the body. Common ways to give chemotherapy include an intravenous (IV) tube placed into a vein or muscle using a needle or in a pill or capsule that is swallowed by mouth. If your child is prescribed oral medications, be sure to ask your health care about how to safely store and handle them at home. A chemotherapy regimen (schedule) usually consists of a specific number of cycles given over a set period of time.
Most children with neuroblastoma will need to have chemotherapy. Chemotherapy may be used as the primary treatment for neuroblastoma. Or, it may be given before surgery to shrink the tumor or after surgery to destroy any remaining cancer cells.
"Induction chemotherapy" means the chemotherapy is being used to destroy as many of the cells as possible to cause the cancer to go into remission. "Consolidation chemotherapy" begins when the child’s cancer has gone into remission.
Children with intermediate-risk neuroblastoma often receive the following drugs:
-
Carboplatin (Paraplatin)
-
Cyclophosphamide (Neosar)
-
Doxorubicin (Adriamycin)
-
Etoposide
Children with high-risk neuroblastoma often receive the following drugs:
-
Busulfan (Busulfex, Myleran)
-
Carboplatin (Paraplatin)
-
Cisplatin (Platinol)
-
Cyclophosphamide (Neosar)
-
Cytokines (GM-CSF and IL2)
-
Dinutuximab (Unituxin)
-
Doxorubicin (Adriamycin, Doxil)
-
Etoposide (VePesid, Toposar)
-
Ifosfamide (Ifex)
-
Isotretinoin
-
Melphalan (Alkeran)
-
Thiotepa
-
Topotecan (Hycamtin)
-
Vincristine (Vincasar)
The side effects of chemotherapy depend on the individual and the dose used, but they can include fatigue, risk of infection, nausea and vomiting, hair loss, loss of appetite, and diarrhea. These side effects usually go away once treatment is finished. The severity of the side effects depends on the type and amount of the drug being given and the length of time the child receives the drug.
Learn more about the basics of chemotherapy and preparing for treatment . The medications used to treat cancer are continually being evaluated. Talking with your child’s doctor is often the best way to learn about the medications prescribed for you, their purpose, and their potential side effects or interactions with other medications. Learn more about your prescriptions by using searchable drug databases .
Return to top
Radiation therapy
Radiation therapy is the use of high-energy x-rays or other particles to destroy cancer cells. A doctor who specializes in giving radiation therapy to treat cancer is called a radiation oncologist. The most common type of radiation treatment is called external-beam radiation therapy, which is radiation given from a machine outside the body.
A radiation therapy regimen, or schedule, usually consists of a specific number of treatments given over a set period of time. Side effects from radiation therapy may include fatigue, mild skin reactions, upset stomach, and loose bowel movements. Most side effects go away soon after treatment is finished. Talk with the radiation oncologist about what side effects your child can expect and how they will be managed.
Because radiation therapy can sometimes cause problems with the normal growth and development of a child’s brain, ovaries, or testicles, the doctor may choose to treat the cancer in another way.
Learn more about the basics of radiation therapy .
Return to top
Stem cell transplantation/bone marrow transplantation
An autologous (AUTO) stem cell transplant is also called a hematopoietic stem cell transplant or bone marrow transplant. This is a medical procedure in which highly specialized cells, called hematopoietic stem cells, are collected from the patient's bloodstream. The stem cells are then frozen until they are needed. Then the patient is given high doses of chemotherapy to treat the neuroblastoma. After that, the collected hematopoietic stem cells, or blood-forming cells, are infused back into the patient.
Before recommending transplantation, doctors will talk with the patient and family about the risks of this treatment. They will also consider several other factors, such as the type of cancer, results of any previous treatment, and the patient’s age and general health.
The goal of transplantation is to destroy cancer cells in the bone marrow, blood, and other parts of the body and allow replacement blood stem cells to create healthy bone marrow. During the process, the patient is treated with high doses of chemotherapy to destroy as many cancer cells as possible. However, this also destroys the patient’s bone marrow tissue and suppresses the immune system. After the high-dose treatment is given, blood stem cells are infused into the patient’s vein to replace the bone marrow and restore normal blood counts.
For neuroblastoma, different combinations of high-dose chemotherapy have been used before the stem cells are infused. A European clinical trial demonstrated that patients who received busulfan (Myleran, Busilvex) and melphalan (Alkeran, Evomela) had improved event-free survival and fewer side effects than children who were treated with carboplatin (Paraplatin), etoposide (available as a generic drug), and melphalan. Event-free survival is the measure of time after treatment that a group of people in a clinical trial has not had their cancer come back or get worse.
At the same time the European study was ongoing, the Children’s Oncology Group conducted a study comparing a single cycle of high-dose chemotherapy (carboplatin, etoposide, and melphalan) with bone marrow transplant, giving tandem cycles of high-dose chemotherapy with bone marrow transplant after each cycle of chemotherapy. The first cycle of consolidation consisted of cyclophosphamide (available as a generic drug) and thiotepa, and the second consisted of carboplatin, etoposide, and melphalan. The results of this Children’s Oncology Group clinical trial demonstrated improved event-free survival for those patients who received 2 cycles of high-dose chemotherapy and stem cell transplant. Based on the results of this study, tandem cycles of high-dose chemotherapy with a stem cell transplant are now considered part of the standard treatment for children with high-risk neuroblastoma.
In an AUTO transplant, there is little risk of tissue rejection because the replacement stem cells are the patient’s own cells, rather than from a donor. However, there is a risk that some of the cells that are put back into the patient could still be cancerous.
Side effects depend on the type of transplant, your child’s general health, and other factors. Learn more about the basics of stem cell and bone marrow transplantation .
Return to top
Immunotherapy
Immunotherapy uses the body's natural defenses to fight cancer by improving your immune system’s ability to attack cancer cells.
GD2 is a compound on the surface of cells, called a disialoganglioside, that is found in large amounts in most neuroblastomas. A variety of monoclonal antibodies directed against GD2 have been used to treat neuroblastoma. A monoclonal antibody is a substance made in a laboratory that acts like the antibodies the body’s immune system naturally makes to fight diseases such as cancer.
The Children's Oncology Group conducted a randomized clinical trial in the early 2000s testing a chimeric (part human, part mouse) anti-GD2 monoclonal antibody therapy (dinutuximab) combined with cytokines (namely, GM-CSF and IL-2), which are proteins that stimulate the immune system, and retinoid therapy (see above) versus retinoic acid alone for patients with high-risk neuroblastoma when:
-
The neuroblastoma responded to induction chemotherapy, and
-
The patient underwent a stem cell transplantation (see above) without the neuroblastoma growing or spreading.
Patients with biopsy-proven residual (leftover) neuroblastoma following a stem cell transplantation were non-randomly assigned to receive immunotherapy as part of the study.
Among the patients enrolled on this clinical trial who were randomized, those who received immunotherapy and RA had higher rates of survival and fewer recurrences than those who received the RA alone. Immunotherapy and RA is now a part of the standard treatment for patients with high-risk neuroblastoma. Based on the results of this study, the U.S. Food and Drug Administration (FDA) approved dinutuximab (Unituxin) in 2015 as part of first-line therapy for children with high-risk neuroblastoma.
In the multi-center SIOPEN clinical trial, the Chimeric14.18 anti-GD2 monoclonal antibody manufactured in animal cells (dinutuximab beta) with or without the IL2 cytokine was evaluated in a randomized clinical trial. The results of this study showed there was no evidence that subcutaneous IL-2 immunotherapy with dinutuximab beta improved event-free survival in patients with high-risk neuroblastoma that responded to standard induction and consolidation treatment. Based on these results, IL-2 is no longer given with dinutuximab in Children's Oncology Group studies, and standard post-consolidation immunotherapy consists of dinutuximab plus GM-CSF and RA.
Humanized versions of the anti-GD2 monoclonal antibody, including naxitamab, a humanized version of the mouse monoclonal anti-GD2 antibody 3F8, and Hu14.18K322A are also being tested in clinical trials. A phase 2 single-institution study of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma showed promising rates of a partial response or better, meaning the tumor had shrunk. In this study, HU14.18K322A was also combined with consolidation treatment and administered following consolidation, and encouraging 3-year event-free survival was observed. A recent Children's Oncology Group pilot study has shown it is feasible to combine dinutuximab with induction chemotherapy in the cooperative group setting. A randomized phase 3 Children's Oncology Group study testing the efficacy of dinutuximab combined with induction chemotherapy is also being developed.
Different types of immunotherapy can cause different side effects. Common side effects include skin reactions, flu-like symptoms, diarrhea, and weight changes. Anti-GD2 antibodies commonly cause severe pain, and narcotics are used to relieve this pain. Other common side effects include low blood pressure, called hypotension, and allergic reactions. Talk with your child’s doctor about what to expect with possible side effects for the immunotherapy recommended for your child. Learn more about the basics of immunotherapy .
Return to top
Retinoid therapy
Retinoids are substances that are similar to vitamin A. They are thought to help some cells mature into normal cells. In a previous Children’s Oncology Group clinical trial, high-risk patients with neuroblastoma who had no evidence of active disease following completion of consolidation therapy received 13-cis-retinoic acid (RA) (isotretinoin) versus no further treatment. The study showed that the children who received RA had improved event-free survival.
Return to top
Targeted therapy ( updated 12/2023 )
Targeted therapy is a treatment that targets the cancer’s specific genes, proteins, or the tissue environment that contributes to cancer growth and survival. This type of treatment blocks the growth and spread of cancer cells while limiting damage to healthy cells.
Not all cancers have the same targets. To find the most effective treatment, your child's doctor may run tests to identify the genes, proteins, and other factors involved in your child's cancer. This helps doctors better match each patient with the most effective treatment whenever possible. In addition, research studies continue to find out more about specific molecular targets and new treatments directed at them. Learn more about the basics of targeted treatments .
Tyrosine kinase inhibitors
Anaplastic lymphoma kinase (ALK) is a normal part of the cell growth process. When present, a change or mutation in the ALK gene helps cancer cells grow. ALK inhibitors help stop this process. ALK mutations have been identified in the germline (inherited) genes of a person and/or the tumor's genes in a subset of patients with neuroblastoma. Drugs that target ALK are called tyrosine kinase inhibitors (TKIs). A number of ALK small-molecule tyrosine kinase inhibitors have been developed and tested in early-phase clinical trials in children when the neuroblastoma continues to grow despite treatment, called refractory disease, or when the neuroblastoma returns following treatment, called recurrent disease.
One study, a phase 3 clinical trial from the Children’s Oncology Group for those with high-risk disease, is testing whether adding the
ALK
inhibitor lorlatinib (Lorbrena) to standard of care treatment helps patients with newly diagnosed neuroblastoma with
ALK
mutations.
Talk with your doctor about all clinical trials that may be available to your child as part of your treatment decision-making. Also, be sure to talk with your doctor about possible side effects from the specific targeted therapy recommended for your child and ways to manage side effects.
Ornithine decarboxylase 1 (ODC1) inhibitor
Ornithine decarboxylase 1 (ODC1) is an important enzyme that contributes to cell survival. In laboratory studies, an ODC inhibitor called eflornithine (Iwilfin, DFMO) slowed the growth of neuroblastoma tumors in mice. In patients with high-risk neuroblastoma, a single-arm phase II study investigated adding eflornithine to treatment after immunotherapy was completed. The results were compared to a control arm of patients who did not receive eflornithine. The analysis showed that patients with high-risk neuroblastoma who received eflornithine after completing immunotherapy had better rates of slowing or stopping the disease and lived longer.
In 2023, the FDA approved eflornithine as treatment to reduce the risk of relapse in adults and children with high-risk neuroblastoma. Eligible patients must have had at least a partial response to previous treatments with multiple medications, including anti-GD2 immunotherapy. This represents the first FDA approval of a medication intended to reduce the risk of relapse in children with high-risk neuroblastoma.
Return to top
Targeted delivery of radionuclides
A radionuclide called 131I MIBG has been used to treat children when the neuroblastoma recurs, and tumor responses have been seen in about 30% of these children. Based on these results, a clinical trial was started by the Children’s Oncology Group testing the effectiveness of using 131I MIBG plus an AUTO stem cell transplant followed by busulfan and melphalan as consolidation therapy for newly diagnosed patients with high-risk disease. The Children’s Oncology Group study is currently conducting a randomized phase 3 clinical trial for this group of patients to test the effectiveness of integrating 131I MIBG in the induction phase of treatment.
Return to top
Physical, emotional, and social effects of cancer
Neuroblastoma and its treatment cause physical symptoms and side effects, as well as emotional, social, and financial effects. Managing all of these effects is called palliative care or supportive care. It is an important part of your child’s care that is included along with treatments intended to slow, stop, or eliminate the cancer.
Palliative care focuses on improving how your child feels during treatment by managing symptoms and supporting patients and their families with other, non-medical needs. Any person, regardless of age or type and stage of cancer, may receive this type of care. And it often works best when it is started right after a cancer diagnosis. People who receive palliative care along with treatment for the cancer often have less severe symptoms, better quality of life, and report that they are more satisfied with treatment.
Palliative treatments vary widely and often include medication, nutritional changes, relaxation techniques, emotional and spiritual support, and other therapies. Your child may also receive palliative treatments similar to those meant to get rid of the cancer, such as cancer medications, surgery, or radiation therapy.
Before treatment begins, talk with your child’s doctor about the goals of each treatment in the recommended treatment plan. You should also talk about the possible side effects of the specific treatment plan and palliative care options. Many patients also benefit from talking with a social worker and participating in support groups. Ask your doctor about these resources, too.
During treatment, your child’s health care team may ask you to answer questions about your child’s symptoms and side effects and to describe each problem. Be sure to tell the health care team if your child is experiencing a problem. This helps the health care team treat any symptoms and side effects as quickly as possible. It can also help prevent more serious problems in the future.
Learn more about the importance of tracking side effects in another part of this guide. Learn more about palliative care in a separate section of this website.
Return to top
Remission and the chance of recurrence
A remission is when a tumor cannot be detected in the body and there are no symptoms. This may also be called having “no evidence of disease” or NED.
A remission may be temporary or permanent. This uncertainty causes many people to worry that their cancer will come back. While many remissions are permanent, it is important to talk with your doctor about the possibility of the tumor returning. Understanding your child’s risk of recurrence and the treatment options may help you and your family feel more prepared if the cancer does return. Learn more about coping with the fear of recurrence .
If neuroblastoma comes back after the original treatment, it is called a recurrent or relapsed cancer. It may come back in the same place (called a local recurrence), nearby (regional recurrence), or in another place (distant recurrence). If there is a recurrence, the cancer may need to be staged again using the system described in Stages and Groups .
If a recurrence happens, a new cycle of testing will begin again to learn as much as possible about it. After this testing is done, you and your child’s doctor will talk about the treatment options. Often the treatment plan will include the treatments described above, such as surgery, cancer medications, and radiation therapy, but they may be used in a different combination or given at a different pace. Your child’s doctor may suggest clinical trials that are studying new ways to treat recurrent neuroblastoma.
Treatment of refractory or recurrent neuroblastoma ( updated 12/2023)
Refractory cancer is cancer that continues to grow despite treatment. The treatment of refractory and recurrent neuroblastoma depends on the location of the previous treatment, tumor biology, the risk group at diagnosis, and whether there are certain gene mutations. There are treatments that work well for patients with low-risk and intermediate-risk disease who have a recurrence where the original tumor started. Recurrent high-risk neuroblastoma remains difficult to treat successfully. Neuroblastoma comes back in approximately 40% to 50% of children with high-risk disease.
In recent years, more treatments and new combinations of treatments have been developed or are being researched for this group of patients. This includes treatment options available through clinical trials. Many of the approaches below are explained above, for additional details.
-
Anti-GD2 antibody immunotherapy. A number of anti-GD2 antibodies have been developed and are being tested in studies for patients with recurrent or refractory neuroblastoma. In a recent Children’s Oncology Group phase 2 clinical trial testing the combination of chemotherapy (irinotecan and temozolomide) plus dinutuximab, tumor responses were seen in about 40% of children with recurrent or refractory neuroblastoma. The Children's Oncology Group is currently conducting a study for patients with recurrent or refractory disease testing the addition of difluoromethylornithine (DFMO) to the chemoimmunotherapy regimen. A European (SIOPEN) BEACON clinical trial is testing the combination of chemotherapy and dinutuximab-beta. Single-institution studies have indicated that, for a subset of patients, some refractory and relapsed high-risk neuroblastoma will respond to the humanized anti-GD2 antibody naxitamab. Promising responses have also been observed with naxitamab combined with temozolomide and irinotecan and GM-CSF in a single-institution phase 2 study. Other anti-GD2 antibodies that have been developed include Hu14.18K332A and dinutuximab-beta. The efficacy of the bispecific antibody Hu3F8-BsAb and the cytokine-antibody fusion molecule Hu14.18-IL2 are also being evaluated. Dinutuxmiab is also being evaluated in combination with natural killer (NK) cells and the immune-modulating agent lenolinomide in a New Approaches to Neuroblastoma Therapy (NANT) phase 1 clinical trial. Dinutuximab combined with 131I-MIBG and vorinostat is also being tested in an ongoing NANT phase 1 clinical trial.
-
Targeted delivery of radionuclides. Radionuclides have been attached to MIBG, as well as somatostatin analogs, which are substances similar to a specific hormone produced by cells. 131I-MIBG therapy has been shown to be active in patients with recurrent or refractory neuroblastoma with response rates of approximately 30%. A recently completed NANT clinical trial testing the efficacy of 131I-MIBG alone or MIBG combined with radiation sensitizers irinotecan or vorinostat (Zolinza) demonstrated higher rates of response with 131I-MIBG combined with vorinostat. Radiosensitizers are drugs that make tumor cells more sensitive to radiation therapy, which makes radiation therapy more effective. Additional studies are now testing the combination of 131I-MIBG and dinutuximab and vorinostat in patients with recurrent or refractory neuroblastoma.
-
Immunotherapies. Early-phase studies testing immune checkpoint inhibitors alone or in combination with MIBG and dinutuximab are ongoing. Additional phase 1 studies are testing T cells engineered to express chimeric antigen receptors (CARs) targeting GD2, LICAM, B7-H3, or other targets. This is called CAR T-cell therapy . Promising responses have also been seen with intrathecal 131I -8H9 (directed against the immune checkpoint B7-H3) in early studies for patients with neuroblastoma that recurs in the brain, in combination with surgery and neuraxis radiation therapy. The efficacy of anti-GD2 vaccines is also being evaluated in patients who achieve a complete response with other treatments in a single-institution study.
-
Therapies that target the ALK oncogene. As explained above, ALK mutations are important targets in the treatment of neuroblastoma. A number of oncogene-specific small-molecule tyrosine kinase inhibitors have been developed, and a phase 1 trial of the ALK inhibitor crizotinib was conducted in the Children’s Oncology Group in children with refractory neuroblastoma or other cancers driven by ALK rearrangements and mutations. Although there were some tumors that respond to this drug, responses in children with ALK- mutated neuroblastoma were less frequent than patients with other types of cancers with ALK mutations. A combination of crizotinib with chemotherapy commonly used in treating newly diagnosed patients with high-risk neuroblastoma has been shown to enhance the sensitivity to ALK inhibition. An ongoing NANT trial is testing a more potent, 3rd generation ALK inhibitor, lorlatinib. Lorlatinib is also being tested in a phase 3 clinical trial in combination with standard of care treatment in patients with newly diagnosed neuroblastoma with ALK mutations. Anti-neuroblastoma activity has also been demonstrated in laboratory studies using an ALK antibody-drug conjugate.
-
Retinoids. Fenretinide (4-HPR) has shown effectiveness against neuroblastoma in a laboratory setting. Newer versions of this drug are in development to make it easier to give this medication to young children.
-
Angiogenesis inhibitors. Anti-angiogenesis therapy is focused on stopping angiogenesis, which is the process of making new blood vessels from existing blood vessels. Because a tumor needs the nutrients delivered by blood vessels to grow and spread, the goal of anti-angiogenesis therapies is to “starve” the tumor. Promising responses have been reported by the European (SIOPEN) BEACON clinical trial in patients with recurrent or refractory disease with temosolomide and topotecan combined with the anti-angiogenic drug bevacizumab.
-
Tyrosine kinase inhibitors. TKIs are a group of drugs that block cell communication and can stop tumor growth. Drugs that inhibit ALK are being tested in clinical trials. Other tyrosine kinase inhibitors that are being tested in clinical trials include inhibitors of the epidermal growth factor receptor (EGFR) and the RAS-MAPK signaling pathway. These receptors help tumor cells grow, and blocking them may slow or stop neuroblastoma growth.
-
Aurora kinase inhibitors. Aurora A kinase helps cells divide early on and is found in all cells that are dividing. Aurora kinase inhibitors are drugs that block this protein, stopping or slowing the cells from dividing. Inhibition of Aurora A kinase also destabilizes the MYCN protein. Early-phase trials testing an Aurora A kinase inhibitor in combination with irinotecan and temozolomide showed promising activity for recurrent neuroblastoma.
-
Combination chemotherapy. The drugs irinotecan (Camptosar) and topotecan are often used early when there is a recurrence. The combination of irinotecan and temozolomide (Methazolastone, Temodar) has few side effects. This chemotherapy treatment regimen has been evaluated in a Children’s Oncology Group study. Some recurrent tumors respond to this treatment. Other chemotherapy regimens have been used when there is a recurrence, including topotecan and low-dose cyclophosphamide.
-
ODC inhibitor. The ODC inhibitor eflornithine is FDA approved as a treatment after immunotherapy to reduce the risk of relapse in adults and children with newly diagnosed high-risk neuroblastoma. Eflornithine is also being tested in early-phase neuroblastoma clinical trials. The Children's Oncology Group is testing the efficacy of the combination of eflornithine and chemotherapy in children with recurrent neuroblastoma.
-
Other treatment options. Demethylating drugs such as decitabine (Dacogen) and histone deacetylase inhibitors, which are substances that can prevent a tumor from growing and spreading, are also being tested for childhood neuroblastoma.
The NANT phase I consortium has a number of open clinical trials testing new treatments for children with recurrent or refractory neuroblastoma. (Please note this link takes you to a separate, independent website.) Several studies are testing combination therapy that includes immunotherapy plus chemotherapy. Always talk with your doctor about all of your child's treatment options, including clinical trials.
Whichever treatment plan you choose, palliative care will be important for relieving symptoms and side effects.
When a child has a recurrent tumor, family members often experience emotions such as disbelief or fear. Families are encouraged to talk with the health care team about these feelings and ask about support services to help them cope. Learn more about dealing with cancer recurrence .
Return to top
If treatment does not work
Although treatment is successful for many children with cancer, sometimes it is not. If a child’s neuroblastoma cannot be cured or controlled, this is called an advanced or terminal cancer. This diagnosis is stressful, and advanced cancer may be difficult to discuss. However, it is important to have open and honest conversations with your child’s health care team to express your family’s feelings, preferences, and concerns. The health care team has special skills, experience, and knowledge to support patients and their families and is there to help.
Hospice care is designed to provide the best possible quality of life for people who are expected to live less than 6 months. Parents and guardians are encouraged to talk with the health care team about hospice options, which include hospice care at home, a special hospice center, or other health care locations. Nursing care and special equipment can make staying at home a workable option for many families.
Some children may be happier and more comfortable if they can attend school part-time or keep up other activities and social connections. The child’s health care team can help parents or guardians decide on an appropriate level of activity. Making sure a child is physically comfortable and free from pain is extremely important as part of end-of-life care. Learn more about caring for a terminally ill child and advanced cancer care planning .
The death of a child is an enormous tragedy, and families may need support to help them cope with the loss. Pediatric cancer centers often have professional staff and support groups to help with the process of grieving. Learn more on grieving the loss of a child .
Return to top
The next section in this guide is About Clinical Trials. It offers more information about research studies that are focused on finding better ways to care for people with cancer. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - About Clinical Trials
ON THIS PAGE: You will learn more about clinical trials, which are the main way that new medical approaches are studied to see how well they work. Use the menu to see other pages.
What are clinical trials?
Doctors and scientists are always looking for better ways to care for children with neuroblastoma. To make scientific advances, doctors create research studies involving volunteers, called clinical trials. Every drug that is now approved by the U.S. Food and Drug Administration (FDA) was tested in clinical trials.
Clinical trials are used for all types and stages of childhood neuroblastoma. Many focus on new treatments to learn if a new treatment is safe, effective, and possibly better than the existing treatments. These types of studies evaluate new drugs, different combinations of existing treatments, new approaches to radiation therapy or surgery, and new methods of treatment. Clinical trials for newly diagnosed patients and for patients with refractory or recurrent disease are ongoing in the Children’s Oncology Group.
Children who participate in clinical trials can be some of the first to get a treatment before it is available to the public. However, there are some risks with a clinical trial, including possible side effects and the chance that the new treatment may not work. Parents are encouraged to talk with their child’s health care team about the pros and cons of joining a specific study.
Some clinical trials study new ways to relieve symptoms and side effects during treatment. Others study ways to manage the late effects that may happen a long time after treatment. Talk with your doctor about clinical trials for symptoms and side effects.
Deciding to join a clinical trial
People decide to participate in clinical trials for many reasons. For some, a clinical trial is the best treatment option available. Because standard treatments are not perfect, patients are often willing to face the added uncertainty of a clinical trial in the hope of a better result. Others volunteer for clinical trials because they know that these studies are a way to contribute to the progress in treating neuroblastoma. Even if they do not benefit directly from the clinical trial, their participation may benefit future children with neuroblastoma.
Insurance coverage and the costs of clinical trials differ by location and by study. In some programs, some of the expenses from participating in the clinical trial are reimbursed. In others, they are not. It is important to talk with the research team and your insurance company first to learn if and how your treatment in a clinical trial will be covered. Learn more about health insurance coverage of clinical trials .
Sometimes people have concerns that, in a clinical trial, their child may receive no treatment by being given a placebo or a “sugar pill.” You and your child will always be told when a placebo is used in a study. Find out more about placebos in cancer clinical trials.
Patient safety and informed consent
To join a clinical trial, parents and children must participate in a process known as informed consent. Informed consent means that parents give permission for their child to participate in a clinical trial and that teenagers give their consent to participate. During informed consent, the doctor should:
-
Describe all of the treatment options so that the person understands how the new treatment differs from the standard treatment.
-
List all of the risks of the new treatment, which may or may not be different from the risks of standard treatment.
-
Explain what will be required of each person in order to participate in the clinical trial, including the number of doctor visits, tests, and the schedule of treatment.
-
Describe the purposes of the clinical trial and what researchers are trying to learn.
Clinical trials also have certain rules called “eligibility criteria” that help structure the research and keep people safe. You and the research team will carefully review these criteria together. Your child will need to meet all of the eligibility criteria in order to participate in a clinical trial. Learn more about eligibility criteria in clinical trials.
People who participate in a clinical trial may stop participating at any time for personal or medical reasons. This may include that the new treatment is not working or there are serious side effects. It is important that parents of a child participating in a clinical trial talk with the doctor and researchers about who will be providing their child’s treatment and care during the clinical trial, after the clinical trials ends, and/or if they choose to leave the clinical trial before it ends.
Finding a clinical trial
Research through clinical trials is ongoing for all types of cancer. For specific topics being studied for neuroblastoma, learn more in the Latest Research section.
Cancer.Net offers more information about cancer clinical trials in other areas of the website, including a complete section on clinical trials and places to search for clinical trials for a specific type of cancer .
In addition, you can find a free video-based educational program about cancer clinical trials located in another section of this website.
The next section in this guide is Latest Research . It explains areas of scientific research for childhood neuroblastoma. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about neuroblastoma and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about neuroblastoma, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials . Always talk with your child’s doctor about the best diagnostic and treatment options for your child.
Many of the items below are explained earlier in this guide's Types of Treatment section. Additional studies are underway to improve the use and effectiveness of current treatments, as well as to find new approaches to treating neuroblastoma.
-
New drug combinations. Clinical trials are underway to study the use of chemotherapy combined with immunotherapy, tyrosine kinase inhibitors, or other drugs. Researchers hope that these drug combinations will increase the effectiveness and decrease the side effects of induction treatment.
-
Stem cell transplantation. A Children’s Oncology Group clinical trial comparing 2 cycles of high-dose chemotherapy to 1 cycle of high-dose chemotherapy and stem cell transplantation has recently been completed. Patients who received tandem cycles of high-dose therapy had improved event-free survival. Based on these results, the Children’s Oncology Group now considers 2 cycles of high-dose therapy with stem cell transplant the new standard of care for high-risk neuroblastoma patients. A European study compared the results for children who received conditioning prior to a stem cell transplant with carboplatin, etoposide, and melphan as compared to busulfan and melphalan. The study suggested that the outcome was better for children treated with busulfan and melphalan.
-
Targeted radiation therapy. Treatment with a high-energy form of radioactive iodine (I-131) with MIBG has been shown to have the tumor respond in about 30% of patients with recurrent or refractory neuroblastoma. This treatment is currently only available at certain cancer centers in the United States. Recently completed New Approaches to Neuroblastoma Therapy (NANT) clinical trials testing radioactive MIBG alone or combined with radiation sensitizers have shown higher response rates when MIBG is combined with vorinostat. Other NANT studies are testing MIBG combined with dinutuximab, and there are plans to amend this study to test vorinostat combined with MIBG and dinutuximab. The Children’s Oncology Group is conducting a randomized phase 3 study evaluating the efficacy of adding radioactive MIBG to the induction regimen in newly diagnosed patients with a high-risk neuroblastoma. Other radiopharmaceutical therapies that are being evaluated include 277 (277Lu)‐DOTATE and astatine‐211 (211At)‐mIBG.
-
Molecular targeted therapies. Research on the use of small molecules to target the cell functions that are abnormal in neuroblastoma cells is ongoing. Lorlatinib, and other drugs that inhibit ALK , a tyrosine kinase that is mutated in a subset of neuroblastomas, as well as other tyrosine kinase inhibitors are being tested in clinical trials. Other treatments currently in phase 1 or phase 2 studies include bromodomain and extra‐terminal motif (BET) bromodomain inhibitors (for their inhibition of MYCN ). Aurora kinase inhibitors have also been combined with chemotherapy for the treatment of neuroblastoma.
-
Biomarkers. New tools are being developed to help find out the clinical aggressiveness of the tumor and response to treatment that may be used in the future to tailor therapies to individual patients. These include the use of circulating tumor DNA for genomic and epigenetic characterization of tumors and for determining how the tumor will respond to treatment. Learn more about tumor marker tests .
-
Imaging techniques. New imaging approaches, such as FDG‐PET‐MRI, 124I‐mIBG PET‐CT, or 68Ga‐DOTATATE, are being evaluated to see how they may help patients with neuroblastoma in the hope that these newer scanning methods may lead to more precise tumor localization.
-
Palliative care/supportive care. Clinical trials are underway to find better ways of reducing symptoms and side effects of current neuroblastoma treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in neuroblastoma, explore these related items that take you outside of this guide:
-
To find clinical trials specific to your child’s diagnosis, talk with your child’s doctor or search online clinical trial databases .
-
Visit the Cancer.Net Blog to review research in neuroblastoma presented at the 2022 ASCO Annual Meeting.
-
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Coping with Treatment
ON THIS PAGE: You will learn more about coping with the physical, emotional, social, and financial effects of childhood cancer and its treatment. Use the menu to see other pages.
Every cancer treatment can cause side effects or changes to your child’s body and how they feel. For many reasons, people do not experience the same side effects even when given the same treatment for the same type of cancer. This can make it hard to predict how your child will feel during treatment.
As your family prepares to start cancer treatment, it is normal to fear treatment-related side effects . It may help to know that your child’s health care team will work to prevent and relieve side effects. This part of cancer treatment is called palliative care or supportive care. It is an important part of your child’s treatment plan, regardless of their age or the stage of disease.
Coping with physical side effects
Common physical side effects from each treatment option for neuroblastoma are described in the Types of Treatment section. Learn more about side effects of cancer and its treatment, along with ways to prevent or control them . Changes to your child’s physical health depend on several factors, including the cancer’s stage, the length and dose of treatment, and your child’s general health.
It is important to discuss any new side effects or changes in existing side effects with your child’s health care team. Providing this information helps them find ways to treat or manage the side effects so your child feels more comfortable and can potentially keep any side effects from worsening.
You may find it helpful to keep track of your child’s side effects so you are prepared to discuss any changes with the health care team. Learn more about why tracking side effects is helpful .
Sometimes, side effects can last after treatment ends. Doctors call these long-term side effects. Side effects that occur months or years after treatment are called late effects . Treating long-term side effects and late effects is an important part of care for childhood cancer survivors. Learn more by reading the Follow-Up Care section of this guide or talking with your child’s doctor.
Coping with emotional and social effects
Your family can have emotional and social effects after a cancer diagnosis. This may include dealing with a variety of emotions, such as anxiety, sadness, or anger, or managing stress. Sometimes, people find it difficult to express how they feel to their loved ones. Some have found that talking to an oncology social worker, counselor, or member of the clergy can help them develop more effective ways of coping and talking about cancer.
You can also find coping strategies for emotional and social effects in a separate section of this website. This section includes many resources for finding support and information to meet your family’s needs.
Coping with the costs of cancer care
Cancer treatment can be expensive. It can be a source of stress and anxiety for families dealing with a cancer diagnosis. In addition to treatment costs, many people find they have extra, unplanned expenses related to their child’s care. Families are encouraged to talk about financial concerns with a member of their health care team. Learn more about managing financial considerations in a separate part of this website.
Coping with barriers to care
Some groups of people experience different rates of new cancer cases and experience different outcomes from their cancer. These differences are called “cancer disparities.” Disparities are caused in part by real-world barriers to quality medical care and social determinants of health , such as where a person lives and whether they have access to food and health care. Cancer disparities more often negatively affect racial and ethnic minorities , people with fewer financial resources , sexual and gender minorities (LGBTQ+) , adolescent and young adult populations , older adults , and people who live in rural areas or other underserved communities
If your child is having difficulty getting the care they need, talk with a member of the health care team or explore other resources that help support medically underserved people .
Talking with your child’s health care team about side effects
Before starting treatment, talk with your child’s doctor about possible side effects. Ask:
-
Which side effects are most likely?
-
When are they likely to happen?
-
What can we do to prevent or relieve them?
-
When and who should we call about side effects?
Be sure to tell your child’s health care team about any side effects that happen during treatment and afterward, too. Tell them even if you do not think the side effects are serious. This discussion should include physical, emotional, social, and financial effects of cancer.
Caring for a child with cancer
Family members and friends often play an important role in taking care of a child with neuroblastoma. This is called being a caregiver. As a parent or guardian, you are the primary caregiver for your child. However, friends and family members can give your family valuable support, even if they live far away.
When your child has neuroblastoma, you may have an additional range of responsibilities. These may include giving medications or managing symptoms and side effects. However, it is important to seek help from others. Below are some of the responsibilities your family or friends could help with:
-
Providing care at home for your child for specific periods of time
-
Giving support and encouragement
-
Assisting with meals or household chores
-
Helping with insurance and billing issues
A caregiving plan can help caregivers stay organized and help identify opportunities to delegate tasks to others. Ask how much care your child may need at home and with daily tasks during and after treatment. Use this 1-page fact sheet that includes an action plan to help make caregiving a team effort. This free fact sheet is available as a PDF, so it is easy to print.
Learn more about caregiving or read the ASCO Answers Guide to Caring for a Loved One With Cancer in English or Spanish .
Looking for More on How to Track Side Effects?
Cancer.Net offers several resources to help you keep track of symptoms and side effects. Please note that these links will take you to other sections of Cancer.Net:
-
Cancer.Net Mobile: The free Cancer.Net mobile app allows you to securely record the time and severity of your child's symptoms and side effects.
-
ASCO Answers Managing Pain: Get this 32-page booklet about the importance of pain relief that includes a pain tracking sheet to record how pain affects your child. The free booklet is available as a PDF, so it is easy to print.
-
ASCO Answers Fact Sheets: Read 1-page fact sheets on anxiety and depression , constipation , diarrhea , rash , and immunotherapy side effects that provide a tracking sheet to record details about the side effect. These free fact sheets are available as PDFs, so they are easy to print, fill out, and give to your health care team.
The next section in this guide is Follow-Up Care. It explains the importance of check-ups after your child finishes cancer treatment. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Follow-Up Care
ON THIS PAGE: You will read about your child’s medical care after treatment is finished and why this follow-up care is important. To see other pages, use the menu.
Care for children diagnosed with cancer does not end when active treatment has finished. Your child’s health care team will continue to check that the cancer has not come back, manage any side effects, and monitor your child’s overall health. This is called follow-up care. All children treated for cancer, including neuroblastoma, should have life-long follow-up care.
Your child’s follow-up care may include regular physical examinations, medical tests, or both. Doctors want to keep track of your child’s recovery in the months and years ahead. Follow-up treatment care for children treated for neuroblastoma mainly depends on the risk grouping:
-
Low-risk or intermediate-risk neuroblastoma. The child is evaluated every 3 to 6 months for 2 years after treatment ends, depending on the treatment given, the child’s age, and other factors. Then, the child is evaluated at least once a year.
-
High-risk, advanced neuroblastoma. Follow-up care is decided on an individual basis. Tests are performed every few months for 2 to 3 years after treatment ends to find out whether the disease has recurred or gotten worse.
Your doctor will create a follow-up care plan for your child. Cancer rehabilitation may be recommended, and this could mean any of a wide range of services such as physical therapy, family or individual counseling, nutritional planning, and/or educational assistance. The goal of rehabilitation is to help survivors and their families regain control over many aspects of their lives and remain as independent as possible. Learn more about cancer rehabilitation.
Learn more about the importance of follow-up care .
Watching for recurrence
One goal of follow-up care is to check for a recurrence, which means that the cancer has come back. Cancer recurs because small areas of cancer cells may remain undetected in the body. Over time, these cells may increase in number until they show up on test results or cause signs or symptoms.
During follow-up care, a doctor familiar with your child’s medical history can give you personalized information about the risk of recurrence. Your doctor will ask specific questions about your child’s health. Some children may have blood tests or imaging tests as part of regular follow-up care. However, testing recommendations depend on several factors, including the type and stage of cancer first diagnosed and the types of treatment given.
The anticipation before having a follow-up test or waiting for test results may add stress to you or a family member. This is sometimes called “scanxiety.” Learn more about how to cope with this type of stress .
Managing long-term and late side effects of childhood cancer
Sometimes, side effects may linger beyond the active treatment period. These are called long-term side effects. In addition, other side effects called late effects may develop months or even years after treatment has ended. Late effects can occur almost anywhere in the body. They include physical problems, such as heart and lung problems, and second cancers, which is a new cancer that happens in someone who has had cancer before. They include emotional problems, such as anxiety and depression, and problems with memory, thinking, attention, and learning.
Based on the type of treatment your child received, the doctor will determine what examinations and tests are needed to check for long-term side effects and the possibility of secondary cancers. Your child’s doctor can recommend the necessary screening tests. Follow-up care should also address the child’s quality of life, including any developmental or emotional concerns. Learn more about the childhood cancer survivorship .
Possible long-term side effects or late effects of neuroblastoma treatment include:
-
Cardiovascular problems. If your child received doxorubicin during chemotherapy, they may be at risk for heart problems , including weakening of the heart muscle. The doctor may recommend imaging of the heart with echocardiograms (echo) or other tests, as well as electrocardiograms (ECG or EKG) and blood pressure monitoring. The risk of these problems is related to the total dose of doxorubicin, but it is also increased if the child received radiation therapy to the chest.
-
Hearing problems. If your child has taken cisplatin or carboplatin, hearing loss is a possible side effect. Hearing tests are recommended at the end of treatment, and then once a year if the test results indicate a hearing problem .
-
Kidney problems. If your child has taken cisplatin or had a bone marrow/stem cell transplant, the doctor will monitor kidney function by doing specific blood and urine tests as a part of a yearly visit. More tests may be needed if test results indicate a problem.
-
Hormonal changes. If your child received radiation therapy, their primary care doctor will monitor growth and development yearly and evaluate your child for delayed puberty starting at age 12 (girls) or 14 (boys) through hormone blood tests.
-
Other cancers. Children diagnosed with neuroblastoma are at increased for developing other cancers, called second cancers . The doctor will monitor your child for subsequent cancers using blood tests and physical exams. More tests may be needed if the test results indicate a problem.
Follow-up care after radiation therapy
Children who have had radiation therapy may be at risk for other cancers, including:
-
Breast cancer. If your child received total body radiation therapy or radiation therapy to the chest, your child should learn how to do a breast self-examination once they reach puberty and perform them monthly. Regular mammograms may begin in early adulthood, rather than waiting until later in life.
-
Skin cancer. You should learn to inspect your child’s skin and ask the doctor to inspect any unusual skin findings at each yearly physical examination.
-
Other cancers. It is important for children with cancer to receive regular primary medical care. Talk with your child’s doctor if you are concerned about any symptoms, especially if your child has ongoing pain or a lump in an area that received radiation therapy.
Follow-up care after stem cell transplantation
Children who have had a stem cell transplantation may have late effects . Possible late effects include:
-
Problems with the way the thyroid gland, kidneys, lungs, and heart work
-
Problems with their body's growth
-
Problems handling infections
-
Increased risk of other cancers
-
Hormone problems and problems with fertility, which is the ability to have a child
-
Hearing loss
Because of these possible problems, it is very important for children treated with stem cell transplantation to have certain tests and immunizations once each year. These may include the following evaluations:
-
Heart tests, such as an echocardiogram and EKG
-
Lung tests, such as pulmonary function testing (PFT)
-
Blood tests that check the function of the kidneys, liver, and thyroid
-
Immunoglobulin levels, such as IgG
-
Hearing tests, if needed
-
Eye examination, if the child received radiation therapy to the head or total body radiation therapy
-
Dental examination
-
Blood tests to evaluate hormone levels, such as testosterone, estrogen, and growth hormones
-
If needed, an examination by an endocrinologist, which is a doctor who specializes in problems with glands and hormones
-
Reproductive organs/gynecologic examination
-
Immunizations as directed by the health care team
Recommendations for long-term follow-up care
The Children's Oncology Group has studied the physical and psychological effects that childhood cancer survivors face. Based on these studies, the Children’s Oncology Group has created recommendations for long-term follow-up care for childhood, adolescent, and young adult cancer survivors that can be found on a separate website: www.survivorshipguidelines.org .
Keeping a child’s personal health record
You are encouraged to organize and keep a personal record of your child’s medical information. The doctor will help you create this. That way, as your child enters adulthood, they have a clear, written history of the diagnosis, the treatments given, and the doctor’s recommendations about the schedule for follow-up care. The American Society of Clinical Oncology (ASCO) offers forms to keep track of the cancer treatment your child received and develop a survivorship care plan when treatment is completed.
Some children continue to see their oncologist, while others transition back to the general care of their pediatrician, primary care doctor, or another health care professional. This decision depends on several factors, including the type and stage of cancer, side effects, health insurance rules, and your family’s personal preferences. Talk with your health care team about your child’s ongoing medical care and any concerns you have about their future health.
If a doctor who was not directly involved in your child’s cancer care will lead the follow-up care, be sure to share the cancer treatment summary and survivorship care plan forms with them and with all future health care providers. Details about the specific cancer treatment given are very valuable to the health care professionals who will care for your child throughout their lifetime.
The next section in this guide is Survivorship . It describes how to cope with challenges in everyday life after a cancer diagnosis. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Survivorship
ON THIS PAGE: You will read about how to cope with challenges in everyday life after your child’s cancer diagnosis. Use the menu to see other pages.
What is survivorship?
The word “survivorship” means different things to different people, but it often describes living with, through, and beyond cancer. In some ways, survivorship is one of the most complicated parts of the cancer experience because it is different for every child and their family.
After active cancer treatment ends, children and their families may experience a mixture of strong feelings, including joy, concern, relief, guilt, and fear. Some people say they appreciate life more after a cancer diagnosis. Other families stay very anxious about their child’s health and become uncertain about coping with everyday life.
One source of stress may occur when frequent visits to the health care team end after completing treatment. Often, relationships built with the cancer care team provide a sense of security during treatment, and children and their families miss this source of support. This may be especially true when new worries and challenges surface over time, such as any late effects of treatment, learning or school problems, emotional challenges, sexual development and fertility concerns, and/or financial issues.
Every family faces different concerns and challenges. With any challenge, a good first step is being able to recognize each fear and talk about it. Effective coping requires:
-
Understanding the challenge your family is facing
-
Thinking through solutions
-
Asking for and allowing the support of others
-
Feeling comfortable with the course of action your family chooses
It may be helpful to join an in-person support group or online community of childhood cancer survivors. Support groups also exist for parents of children diagnosed with cancer. This allows you to talk with people who have had similar first-hand experiences. Other options for finding support include talking with a friend or member of the health care team, individual counseling, or asking for assistance at the learning resource center of the place where your child received treatment.
Healthy living after cancer
Survivorship often serves as a strong motivator to make lifestyle changes, often for the whole family. Children who have had cancer can enhance the quality of their future by following established guidelines for good health into and through adulthood, including not smoking, limiting alcohol, maintaining a healthy weight, eating well, managing stress, and participating in regular physical activity. Talk with the doctor about developing a plan that is best for your child’s needs. Learn more about making healthy lifestyle choices .
It is important that your child has recommended medical checkups and tests (see Follow-Up Care ) to take care of their health.
Talk with the doctor to develop a survivorship care plan that is best for your child’s needs.
Changing role of caregivers
Parents, other family members, and friends may also go through periods of transition. A caregiver plays a very important role in supporting a child diagnosed with cancer, providing physical, emotional, and practical care on a daily or as-needed basis. Many caregivers become focused on providing this support, especially if the treatment period lasts for many months or longer.
However, as treatment is completed, the caregiver's role often changes. Eventually, the need for caregiving related to a child’s cancer diagnosis will become much less or come to an end as your child gets older. Family counselors at a pediatric cancer center can help with this transition. You can also learn more about adjusting to life after caregiving.
Looking for More Survivorship Resources?
For more information about cancer survivorship, explore these related items. Please note these links will take you to other sections of Cancer.Net:
-
Survivorship Resources: Cancer.Net offers information and resources to help survivors cope, including specific sections for children and teens and young adults . There is also a main section on survivorship for people of all ages.
-
ASCO Answers Guide to Cancer Survivorship: Get this 48-page booklet that helps people transition into life after treatment. It includes blank treatment summary and survivorship care plan forms. The free booklet is available as a PDF, so it is easy to print out.
The next section offers Questions to Ask the Health Care Team to help start conversations with your child’s cancer care team. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Questions to Ask the Health Care Team
ON THIS PAGE: You will find some questions to ask your child’s doctor or other members of the health care team, to help you better understand your child’s diagnosis, treatment plan, and overall care. Use the menu to see other pages.
Talking often with the health care team is important to make informed decisions about your child’s health care. These suggested questions are a starting point to help you learn more about your cancer care and treatment. You are also encouraged to ask additional questions that are important to you. You may want to print this list and bring it to your child’s next appointment, or download Cancer.Net’s free mobile app for a digital list and other interactive tools to manage your child’s care. It may also be helpful to ask a family member or friend to come with you to appointments to help take notes.
Questions to ask after getting a diagnosis
-
Where is the cancer located?
-
Can you explain my child’s pathology report (laboratory test results) to me?
-
What is the stage of the cancer? What does this mean?
-
What risk group has my child’s neuroblastoma been classified as? What does this mean?
-
Does my child’s tumor have any tumor markers?
Questions to ask about choosing a treatment and managing side effects
-
What are my child’s treatment options?
-
What clinical trials are available for my child? Where are they located, and how do I find out more about them?
-
Does this hospital participate in clinical trials for children with neuroblastoma?
-
What treatment plan do you recommend? Why?
-
What is the goal of each treatment? Is it to eliminate the cancer, help my child feel better, or both?
-
What is my child’s prognosis?
-
How many children with neuroblastoma are seen and treated at this hospital?
-
Who will be part of my child’s health care team, and what does each member do?
-
Who will be leading my child’s overall treatment?
-
What are the possible side effects of this treatment, both in the short term and the long term?
-
How will this treatment affect my child’s daily life? Will they be able to attend school and perform their usual activities?
-
Could this treatment affect my child’s ability to become pregnant or have children? If so, should my family talk with a fertility specialist before cancer treatment begins?
-
If I’m worried about managing the costs of medical care, who can help me?
-
What follow-up tests are needed, and how often are they needed?
-
What support services are available to my child? To my family?
-
If I have questions or problems, who should I call?
Questions to ask about having surgery
-
What type of surgery will my child have? Will lymph nodes be removed?
-
How long will the operation take?
-
How long will my child be in the hospital?
-
Can you describe what my child’s recovery from surgery will be like?
-
Who should I contact about any side effects my child experiences? And how soon?
-
What are the possible long-term effects of having this surgery?
-
Will other treatments be needed after this surgery?
Questions to ask when radiation therapy is recommended
-
Why is this treatment being recommended?
-
What is the goal of this treatment?
-
How long will it take to give each treatment?
-
Will my child receive this treatment at a hospital or clinic?
-
What side effects can my child expect during each treatment?
-
Who should I contact about any side effects my child experiences? And how soon?
-
What are the possible long-term or late effects of having this treatment?
-
What can be done to prevent or relieve the side effects?
-
Will my child need tests or scans during this treatment period?
Questions to ask when chemotherapy, targeted therapy, or immunotherapy is recommended
-
What specific treatment(s) are recommended for my child?
-
Why are these other types of treatment being recommended?
-
What is the goal of each treatment?
-
How long will it take to give each treatment?
-
Will my child receive this treatment at a hospital or clinic? Or will they take it at home?
-
What side effects can my child expect during each treatment?
-
Who should I contact about any side effects my child experiences? And how soon?
-
What are the possible long-term or late effects of having each treatment?
-
What can be done to prevent or relieve the side effects?
-
Will my child need tests or scans during this treatment period?
Questions to ask when stem cell/bone marrow transplantation is recommended
-
Why is this treatment being recommended?
-
What is the goal of this treatment?
-
How long will it take to give each treatment?
-
Will my child receive this treatment at a hospital or clinic? Or will they take it at home?
-
What side effects can my child expect during each treatment?
-
Who should I contact about any side effects my child experiences? And how soon?
-
What are the possible long-term or late effects of having each treatment?
-
What can be done to prevent or relieve the side effects?
-
Will my child need tests or scans during this treatment period?
Questions to ask about planning follow-up care
-
What is the chance that the cancer will come back? Should I watch for specific signs or symptoms?
-
What long-term side effects or late effects are possible based on the cancer treatment my child received?
-
What follow-up tests will my child need, and how often will those tests be needed?
-
How do I get a treatment summary and survivorship care plan to keep in my child’s personal records?
-
Who will be leading my child’s follow-up care?
-
What survivorship support services are available to my child? To my family?
The next section in this guide is Additional Resources . It offers more resources on this website that may be helpful to you. Use the menu to choose a different section to read in this guide.
Neuroblastoma - Childhood - Additional Resources
ON THIS PAGE: You will find some helpful links to other areas of Cancer.Net that provide information about cancer care and treatment. This is the final page of Cancer.Net’s Guide to Childhood Neuroblastoma. Use the menu to go back and see other pages.
Cancer.Net includes many other sections about the medical and emotional aspects of a tumor, for the person diagnosed and their family members and friends. This website is meant to be a resource for you and your loved ones from the time of diagnosis, through treatment, and beyond.
Here are a few links to help you explore other parts of Cancer.Net:
-
Search for a cancer specialist in your local area using this free database of doctors from the American Society of Clinical Oncology.
-
Learn what medical phrases and terms used in cancer care and treatment mean.
-
Read more about the first steps to take when your child is diagnosed with cancer .
-
Find out more about clinical trials as a treatment option.
-
Get information about managing the financial costs of cancer care.
-
Learn more about coping with the emotions that cancer can bring, including those within a family or a relationship.
-
Find a national, not-for-profit advocacy organization that may offer additional information, services, and support for families coping with childhood neuroblastoma.
-
Explore what to do when your child finishes active treatment .
-
Download Cancer.Net Mobile , a free app that includes a symptom and side effect tracker, medication reminders, and other interactive resources.
-
To find a range of information and insights from different voices on timely cancer topics, visit the Cancer.Net Blog .
-
Watch Cancer.Net videos with ASCO experts explaining the basics of cancer treatment, side effects, survivorship, clinical trials, caregiving, and more.
This is the end of Cancer.Net’s Guide to Childhood Neuroblastoma. Use the menu to choose a different section to read in this guide.













